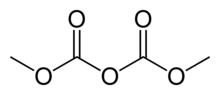
Chemistry:Dimethyl dicarbonate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dimethyl dicarbonate | |
| Other names
DMDC; Dicarbonic acid dimethyl ester; Dimethyl pyrocarbonate; Velcorin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H6O5 | |
| Molar mass | 134.087 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.25 g/mL |
| Melting point | 16 to 18 °C (61 to 64 °F; 289 to 291 K) |
| Boiling point | 172 °C (342 °F; 445 K) |
| Viscosity | 2.1 Pa·s (20 °C) |
| Hazards | |
| Main hazards | Toxic |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H226, H302, H312, H314, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P363 | |
| Related compounds | |
Related compounds
|
Di-tert-butyl dicarbonate diethylpyrocarbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyl dicarbonate (DMDC) is a colorless liquid and a pungent odor at high concentration at room temperature. It is primarily used as a beverage preservative, processing aid, or sterilant (INS No. 242) being highly active against typical beverage spoiling microorganisms like yeast, bacteria, or mould.[1]
Usage
Dimethyl dicarbonate is used to stabilize beverages by preventing microbial spoilage. It can be used in various non-alcoholic as well as alcoholic drinks like wine, cider, beer-mix beverages or hard seltzers. Beverage spoiling microbes are killed by methoxycarbonylation of proteins.
It acts by inhibiting enzymes involved in the microbial metabolism, e.g. acetate kinase and L-glutamic acid decarboxylase.[2] It has also been proposed that DMDC inhibits the enzymes alcohol dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase by causing the methoxycarbonylation of their histidine components.[3]
In wine, it is often used to replace potassium sorbate, as it inactivates wine spoilage yeasts such as Brettanomyces. Once it has been added to beverages, the efficacy of the chemical is provided by the following reactions:
- DMDC + water → methanol + carbon dioxide
- DMDC + ethanol → ethyl methyl carbonate
- DMDC + ammonia → methyl carbamate
- DMDC + amino acid → derived carboxymethyl
The application of DMDC is particularly useful when wine needs to be sterilized but cannot be sterile filtered, pasteurized, or sulfured. DMDC is also used to stabilize non-alcoholic beverages such as carbonated or non-carbonated juice beverages, isotonic sports beverages, iced teas and flavored waters. DMDC is produced by Lanxess under the trade name Velcorin®
DMDC is added before the filling of the beverage. It then breaks down into small amounts of methanol and carbon dioxide, which are both natural constituents of fruit and vegetable juices.
The EU Scientific Committee on Food, the FDA in the United States and the JECFA of the WHO have confirmed the safe use in beverages. The FDA approved its use in wines in 1988, with the maximum level being permitted set at 200 mg/L, and only if there were fewer than 500 yeast cells/mL at time of dosage.[4] It is also approved in the EU, where it is listed under E number E242,[5] as well as Australia and New Zealand.[6]
See also
References
- ↑ Uhr, Hermann; Mielke, Burkhard; Exner, Otto; Payne, Ken R.; Hill, Edward (2013). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–26. doi:10.1002/14356007.a16_563.pub2.
- ↑ [1][|permanent dead link|dead link}}]
- ↑ DMDC's role in bottle stability - dimethyl dicarbonate , Wines & Vines, Oct 1990
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part I". Archived from the original on 2013-03-14. https://web.archive.org/web/20130314104055/https://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/FoodAdditiveListings/ucm091048.htm. Retrieved 2011-10-27.
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist. Retrieved 2011-10-27.
- ↑ Food Standards Australia and New Zealand, Application A1015 - Classification of Dimethyl Dicarbonate - Approval Report -15 December 2010"Application A1025 - Classification of Dimethyl Dicarbonate". http://www.foodstandards.gov.au/code/applications/pages/applicationa1025clas4387.aspx. Retrieved 2010-12-15.
External links
- Dimethyl dicarbonate at EPA SRS
- Dimethyl dicarbonate technical data at FAO
- Dimethyl dicarbonate and microbiological stability
 |
