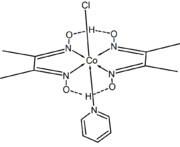Chemistry:Dimethylglyoxime
| |
| |
| Names | |
|---|---|
| IUPAC name
N,N′-Dihydroxy-2,3-butanediimine
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C4H8N2O2 | |
| Molar mass | 116.120 g·mol−1 |
| Appearance | White/Off White Powder |
| Density | 1.37 g/cm3 |
| Melting point | 240 to 241 °C (464 to 466 °F; 513 to 514 K) |
| Boiling point | decomposes |
| low | |
| Structure | |
| 0 | |
| Hazards | |
| Main hazards | Toxic, Skin/Eye Irritant |
| Safety data sheet | External MSDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H228, H301 | |
| P210, P240, P241, P264, P270, P280, P301+310, P321, P330, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Hydroxylamine salicylaldoxime |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
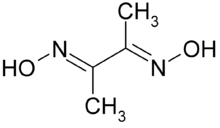
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH− for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.
Preparation
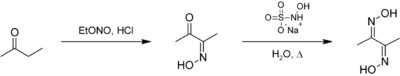
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:[1]
Complexes
Dimethylglyoxime forms complexes with metals including nickel,[2] palladium and cobalt.[3] These complexes are used to separate those cations from solutions of metal salts and in gravimetric analysis. It is also used in precious metals refining to precipitate palladium from solutions of palladium chloride.
Reaction of Ni-dmg-Formation
Sample of Ni(dmgH)2
References
- ↑ Semon, W. L.; Damerell, V. R. (1930). "Dimethylglyoxime". Organic Syntheses 10: 22. doi:10.15227/orgsyn.010.0022.
- ↑ Lev Tschugaeff (1905). "Über ein neues, empfindliches Reagens auf Nickel". Berichte der Deutschen Chemischen Gesellschaft 38 (3): 2520–2522. doi:10.1002/cber.19050380317. https://zenodo.org/record/1426158.
- ↑ Girolami, G. S.; Rauchfuss, T.B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual (3rd ed.). pp. 213–215.
 |