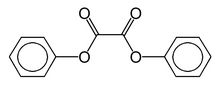
Chemistry:Diphenyl oxalate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Diphenyl oxalate | |
| Other names
diphenylethandioate, oxalic acid diphenyl ester, cyalume, DPO
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C14H10O4 | |
| Molar mass | 242.227 g/mol |
| Appearance | solid |
| Melting point | 136 °C (277 °F; 409 K) |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
129·0 ± 0·8[1] |
| Related compounds | |
Related compounds
|
Dimethyl oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
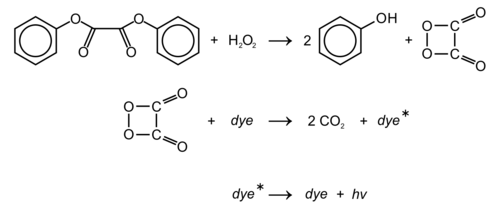
Diphenyl oxalate (trademark name Cyalume) is a solid whose oxidation products are responsible for the chemiluminescence in a glowstick. This chemical is the double ester of phenol with oxalic acid. Upon reaction with hydrogen peroxide, 1,2-dioxetanedione is formed, along with release of the two phenols.[2] The dioxetanedione then reacts with a dye molecule, decomposing to form carbon dioxide and leaving the dye in an excited state. As the dye relaxes back to its unexcited state, it releases a photon of visible light.
The reaction rate is pH dependent, and slightly alkaline conditions, achieved by adding a weak base, such as sodium salicylate, give a faster reaction and therefore produce brighter light. The 2,4,6-trichlorophenol ester is a solid and thus easier to handle.[clarification needed] Furthermore, since trichlorophenolate is the better leaving group, the reaction will proceed faster, again producing brighter light, as compared to the phenol ester.
The following colors can be produced by using different dyes:
| Color | Compound |
|---|---|
| Green | 9,10-Diphenylanthracene |
| Blue | 9,10-Bis(phenylethynyl)anthracene |
| Yellow-green | Tetracene |
| Yellow | 1-Chloro-9,10-bis(phenylethynyl)anthracene |
| Orange | 5,12-Bis(phenylethynyl)naphthacene, Rubrene, Rhodamine 6G |
| Red | Rhodamine B |
References
- ↑ Carson, A. S.; Fine, D. H.; Gray, P.; Laye, P. G. (1971). "Standard enthalpies of formation of diphenyl oxalate and benzoic anhydride and some related bond dissociation energies". Journal of the Chemical Society B: Physical Organic: 1611. doi:10.1039/J29710001611.
- ↑ Orosz, György (January 1989). "The role of diaryl oxalates in peroxioxalate chemiluminescence". Tetrahedron 45 (11): 3493–3506. doi:10.1016/S0040-4020(01)81028-0.
 |