Chemistry:Enterocin
| |
| Names | |
|---|---|
| IUPAC name
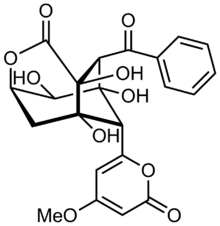
(10S)-2-benzoyl-1,3,8,10-tetrahydroxy-9-(4-methoxy-6-oxopyran-2-yl)-5-oxatricyclo[4.3.1.03,8]decan-4-one
| |
| Other names
Vulgamycin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C22H20O10 | |
| Molar mass | 444.392 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Enterocin and its derivatives are bacteriocins synthesized by the lactic acid bacteria, Enterococcus. This class of polyketide antibiotics are effective against foodborne pathogens including L. monocytogenes, Listeria, and Bacillus.[1] Due to its proteolytic degradability in the gastrointestinal tract, enterocin is used for controlling foodborne pathogens via human consumption.[2]
History
Enterocin was discovered from soil and marine Streptomyces[3] strains as well as from marine ascidians of Didemnum[4] and it has also been found in a mangrove strains Streptomyces qinglanensis and Salinispora pacifica.[5]
Total synthesis
The total synthesis of enterocin has been reported.[6]
Biosynthesis
Enterocin has a caged, tricyclic, nonaromatic core and its formation undergoes a flavoenzyme (EncM) catalyzed Favorskii-like rearrangement of a poly(beta-carbonyl).[7] Studies done on enterocin have shown that it is biosynthesized from a type II polyketide synthase (PKS) pathway, starting with a structure derived from phenylalanine or activation of benzoic acid followed by the EncM catalyzed rearrangement.
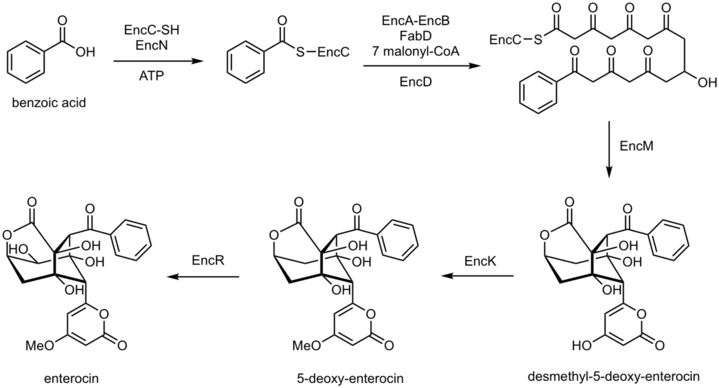
The enzyme EncN catalyzes the ATP-dependent transfer of the benzoate to EncC, the acyl carrier protein. EncC transfers the aromatic unit to EncA-EncB, the ketosynthase in order for malonation via FabD, the malonyl-CoA:ACP transacylase. A Claisen condensation occurs between the benzoyl and malonyl groups and occurs six more times followed by reaction with EncD, a ketoreductase; the intermediate undergoes the EncM catalyzed oxidative rearrangement to form the enterocin tricyclic core. Further reaction with O-methyltransferase, EncK and cytochrome P450 hydroxylase, EncR yields enterocin.[9]
References
- ↑ "Enterocins in food preservation". International Journal of Food Microbiology 141 (1–2): 1–10. June 2010. doi:10.1016/j.ijfoodmicro.2010.03.005. PMID 20399522.
- ↑ "Fresh-Cut Fruits: Microbial Degradation and Preservation". Microbial Contamination and Food Degradation. 2018-01-01. pp. 149–176. doi:10.1016/B978-0-12-811515-2.00006-8. ISBN 978-0-12-811515-2.
- ↑ "Enterocin, a new antibiotic taxonomy, isolation and characterization". The Journal of Antibiotics 29 (3): 227–35. March 1976. doi:10.7164/antibiotics.29.227. PMID 770404.
- ↑ "Isolation of Microbial Antibiotics from a Marine Ascidian of the GenusDidemnum". The Journal of Organic Chemistry 61 (4): 1543–1546. 1996. doi:10.1021/jo951794g. ISSN 0022-3263. https://pubs.acs.org/doi/pdf/10.1021/jo951794g.
- ↑ "Direct capture and heterologous expression of Salinispora natural product genes for the biosynthesis of enterocin". Journal of Natural Products 78 (3): 539–42. March 2015. doi:10.1021/np500664q. PMID 25382643.
- ↑ "Toward (-)-Enterocin: An Improved Cuprate Barbier Protocol To Overcome Strain and Sterical Hindrance". Organic Letters 20 (7): 1841–1844. April 2018. doi:10.1021/acs.orglett.8b00353. PMID 29553746.
- ↑ "Flavin-mediated dual oxidation controls an enzymatic Favorskii-type rearrangement". Nature 503 (7477): 552–556. November 2013. doi:10.1038/nature12643. PMID 24162851. Bibcode: 2013Natur.503..552T.
- ↑ "Type II PKS". Comprehensive Natural Products II. 2010-01-01. pp. 227–303. doi:10.1016/B978-008045382-8.00703-6. ISBN 9780080453828.
- ↑ "In vitro biosynthesis of unnatural enterocin and wailupemycin polyketides". Journal of Natural Products 72 (3): 469–72. March 2009. doi:10.1021/np800598t. PMID 19215142.
 |

