Chemistry:Fluorine perchlorate
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Perchloryl hypofluorite
| |||
| Other names
Fluorine perchlorate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| FClO4 | |||
| Melting point | −167.3 °C (−269.1 °F; 105.8 K) | ||
| Boiling point | −16 °C (3 °F; 257 K) | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
9 kcal/mol[1] | ||
| Hazards | |||
| Main hazards | Highly explosive gas | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
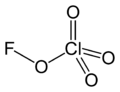
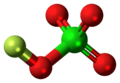
Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO4F or FOClO3. It is an extremely unstable gas that explodes spontaneously[2] and has a penetrating odor.[3]
Synthesis
One synthesis uses fluorine and perchloric acid,[4] though the action of ClF5 on water is another method.[citation needed]
- [math]\ce{ F2 + HClO4 -> FClO4 + HF }[/math]
Another method of synthesis involves the thermal decomposition of tetrafluoroammonium perchlorate, NF4ClO4, which yields very pure FClO4 that may be manipulated and frozen without explosions.[5]
- [math]\ce{ NF4ClO4 ->[\ \Delta\ ] NF3{} + FClO4 }[/math]
Structure
Fluorine perchlorate is not analogous to perchloric acid because the fluorine atom is more electronegative than oxygen. It contains an oxygen atom in a rare oxidation state of 0 due to the electronegativity of oxygen, which is higher than that of chlorine but lower than that of fluorine.
Safety
FClO4 has a very dangerous and unpredictable series of reactions associated with it, as a covalent perchlorate (chlorine in the +7 oxidation state) and a compound featuring a very sensitive O-F single bond. Small amounts of reducing agent, such as organic compounds, can trigger explosive detonation. Products of these decomposition reactions could include oxygen halides, interhalogen compounds, and other hazardous substances.
Accidental synthesis is possible if precursors are carelessly mixed. Like similar covalent fluorides and perchlorates, it needs to be handled with extreme caution.
Reaction
FClO4 is a strong oxidant and it reacts with iodide ion:
- [math]\ce{ FOClO3 + 2I^- -> ClO4^- + F^- + I2 }[/math]
FClO4 can also react with tetrafluoroethylene:[6]
- [math]\ce{ CF2=CF2 + FOClO3 -> CF3CF2OClO3 }[/math]
It may be a radical addition reaction.[7]
References
- ↑ Breazeale, J. D.; MacLaren, R. O.. Thermochemistry of oxygen-fluorine bonding, United Technology Center, Sunnyvale, CA, 1963. Accession Number: AD0402889. Retrieved online from [1] on 2009-05-21.
- ↑ Pradyot Patnaik. A comprehensive guide to the hazardous properties of chemical substances, 3rd ed., Wiley-Interscience, 2007. ISBN:0-471-71458-5
- ↑ Robert Alan Lewis. Lewis' dictionary of toxicology, CRC Press, 1998, p. 508. ISBN:1-56670-223-2
- ↑ Rohrback, G. H.; Cady, G. H. (1947). "The Preparation of Fluorine Perchlorate from Fluorine and Perchloric Acid". Journal of the American Chemical Society 69 (3): 677–678. doi:10.1021/ja01195a063.
- ↑ Schack, C. J.; Christe, K. O. (1979). "Reactions of fluorine perchlorate with fluorocarbons and the polarity of the oxygen-fluorine bond in covalent hypofluorites". Inorganic Chemistry 18 (9): 2619–2620. doi:10.1021/ic50199a056.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Schack, Carl J.; Christe, Karl O. (1979). "Reactions of fluorine perchlorate with fluorocarbons and the polarity of the oxygen-fluorine bond in covalent hypofluorites". Inorganic Chemistry 18 (9): 2619. doi:10.1021/ic50199a056.
External links
- Chemist Derek Lowe's experiences with perchlorates
 |