Chemistry:Chlorine pentafluoride
|
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| ClF5 | |||
| Molar mass | 130.445 g mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 4.5 kg/m3 (g/L) | ||
| Melting point | −103 °C (−153 °F; 170 K) | ||
| Boiling point | −13.1 °C (8.4 °F; 260.0 K) | ||
| Hydrolyzes | |||
| Structure | |||
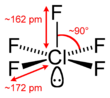
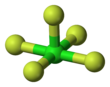
| Square pyramidal | |||
| Thermochemistry | |||
Std molar
entropy (S |
310.73 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−238.49 kJ mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless liquid is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry,[1] as confirmed by its high-resolution 19F NMR spectrum.[2] It was first synthesized in 1963.[3]
Preparation
Some of the earliest research on the preparation was classified.[4][5] It was first prepared by fluorination of chlorine trifluoride at high temperatures and high pressures:[4]
- ClF3 + F2 → ClF5
- ClF + 2F2 → ClF5
- Cl2 + 5F2 → 2ClF5
- CsClF4 + F2 → CsF + ClF5
NiF2 catalyzes this reaction.[6]
Certain metal fluorides, MClF4 (i.e. KClF4, RbClF4, CsClF4), react with F2 to produce ClF5 and the corresponding alkali metal fluoride.[5]
Reactions
In a highly exothermic reaction, ClF5 reacts with water to produce chloryl fluoride and hydrogen fluoride:[7]
- ClF5 + 2 H2O → ClO2F + 4 HF
It is also a strong fluorinating agent. At room temperature it reacts readily with all elements (including otherwise "inert" elements like platinum and gold) except noble gases, nitrogen, oxygen and fluorine.[2]
Uses
Rocket propellant
Chlorine pentafluoride was once considered for use as an oxidizer for rockets. As a propellant, it has a higher maximum specific impulse than ClF3, but with the same difficulties in handling.[4] Due to the hazardous nature of chlorine pentafluoride, it has yet to be used in a large scale rocket propulsion system.
See also
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 833. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 Pilipovich, D.; Maya, W.; Lawton, E.A.; Bauer, H.F.; Sheehan, D. F.; Ogimachi, N. N.; Wilson, R. D.; Gunderloy, F. C. et al. (1967). "Chlorine pentafluoride. Preparation and Properties". Inorganic Chemistry 6 (10): 1918. doi:10.1021/ic50056a036.
- ↑ Smith D. F. (1963). "Chlorine Pentafluoride". Science 141 (3585): 1039–1040. doi:10.1126/science.141.3585.1039. PMID 17739492. Bibcode: 1963Sci...141.1039S.
- ↑ 4.0 4.1 4.2 Clark, John D. (1972). Ignition! An Informal History of Liquid Rocket Propellants. Rutgers University Press. pp. 87–88. ISBN 0-8135-0725-1. http://www.sciencemadness.org/library/books/ignition.pdf.
- ↑ 5.0 5.1 Smith D. F. (1963). "Chlorine Pentafluoride". Science 141 (3585): 1039–1040. doi:10.1126/science.141.3585.1039. PMID 17739492. Bibcode: 1963Sci...141.1039S.
- ↑ "On the Synthesis of Chlorine Pentafluoride". Journal of Fluorine Chemistry 17 (4): 381–383. 1981. doi:10.1016/S0022-1139(00)81783-2.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 834. ISBN 978-0-08-037941-8.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- New Jersey Hazardous Substance Fact Sheet
- WebBook page for ClF5
 |