Chemistry:Francium hydroxide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
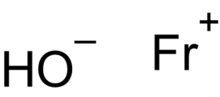
| FrOH | |
| Molar mass | 240 g·mol−1 |
| Related compounds | |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Francium hydroxide is a hypothetical inorganic compound with a chemical formula FrOH. It is a hydroxide of francium.
It probably can be produced by reacting francium metal with water:[1]
- 2 Fr + 2 H
2O → 2 FrOH + H
2 (gas)
This reaction might be explosive, because this reaction is probably very exothermic, because of which water could suddenly start boiling violently, producing hot water vapor, and very flammable hydrogen gas is produced in the reaction as well, and hydrogen could ignite, causing fire and explosion. However this is all guesswork, as a visible quantity of francium has never been made.
Francium hydroxide's alkalinity is predicted to be stronger than caesium hydroxide.[2]
References
- ↑ Robert Krebs (2006), "Francium" (in en), The History and Use of Our Earth's Chemical Elements: A Reference Guide, Robert Krebs, p. 64, ISBN 0313334382, https://books.google.com/books?id=yb9xTj72vNAC&pg=PA64
- ↑ Douglas Considine, Glenn Considine: Van Nostrand’s Scientific Encyclopedia., ISBN:978-1-4757-6920-3, s. 605
 |

