Chemistry:Geniposide
| |
| Names | |
|---|---|
| IUPAC name
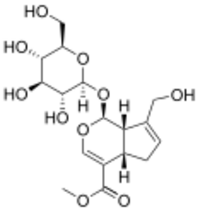
Methyl (1S,4aS,7aS)-1-(β-D-glucopyranosyloxy)-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate
| |
| Systematic IUPAC name
Methyl (1S,4aS,7aS)-7-(hydroxymethyl)-1-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate | |
| Other names
Jasminoidin;[1] methyl 1-(hexopyranosyloxy)-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | geniposide |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H24O10 | |
| Molar mass | 388.369 g·mol−1 |
| Melting point | 245.23 °C (473.41 °F; 518.38 K) |
| Boiling point | 641.4±55.0 °C at 760 mmHg |
| log P | -1.854 |
| Acidity (pKa) | 12.80±0.70 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H301 | |
| P264, P270, P301+310, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Geniposide, the glycoside form of genipin, is a bioactive iridoid glycoside that is found in a wide variety of medicinal herbs, such as Gardenia jasminoides (fruits) .[2] Geniposide shows several pharmacological effects (in vitro and in vivo) including neuroprotective, antidiabetic, hepatoprotective, anti-inflammatory, analgesic, antidepressant-like, cardioprotective, antioxidant, immune-regulatory, antithrombotic and antitumoral activity.[3] These pharmacology benefits arise through the modulating action of geniposide on several proteins and genes that are associated with inflammatory and oxidative stress processes.[4]
Physiological activity
Neuroprotective
A growing body of evidence shows that the neuroprotective benefit of geniposide probably arises from its agonist action on the glucagon-like peptide-1(GLP-1R) receptor. When this receptor is activated neurotrophic effects were induced in cells, such as neurite outgrowth, reducing amyloid plaques, inhibiting τ phosphorylation, preventing memory impairment and loss of synapses, reducing oxidative stress and the chronic inflammatory response.,[5][6] These effects could be promising in the treatment of Alzheimer's and Parkinson's diseases.
Antidepressant-Like
Studies on depressive rats (induced by chronic unpredictable mild stress) have shown that the antidepressant effect of geniposide is similar to fluoxetine. This effect could be mediated by geniposide's effects on the hypothalamus–pituitary–adrenal (HPA) axis, whose dysfunction has been associated with the pathogenesis of depression. In a depressive state the serum levels of adrenocorticotropic hormone and cortisol are increased, as well as hypothalamic corticotropin-releasing hormone gene expression. The dysfunction of the HPA axis plays an important rule in the serotonergic system. When HPA axis shows high levels of activity, this leads to a hyper secretion of cortisol and corticotropin-releasing hormone, in the opposite case the serotonergic system suffers a downregulation and decreases its activity. Low serotonergic system activity results in hyporesponsivity of 5-hydroxytryptamine (5-HT) in the hippocampus.[7][8] Geniposide was able to reverse the high levels of cortisol and hypothalamic corticotropin-releasing hormone gene expression, which lead to an increase of 5-HT in the hippocampus and 5-Hydroxyindoleacetic acid (5-HIAA) in striatum.[7]
Antioxidant
Geniposide shows a reasonable capacity of induction of endogenous antioxidative proteins, which offer protection against cell injury by oxidative stress. A study with hippocampal neurons revealed that geniposide could enhance cytoprotection, though the activation of the enzyme Phosphoinositide 3-kinases (PI3K) and the induction of the nuclear translocation of erythroid 2–related factor 2 (NFE2L2).[9] The PI3K/Nrf2 pathway signaling triggers several responses, such as the expression of antioxidative enzymes heme oxygenase (HO-1), superoxide dismutase (SOD) and NAD(P)H dehydrogenase quinone 1 (NQO1), reducing accumulation of reactive oxygen species (ROS).[10]
Anti-inflammatory
Several studies have shown geniposide's potential to treat inflammatory diseases, such as arthritis, due to its effect in the production on cytokine and pro-inflammatory mediators. In rats with arthritis, oral administration of geniposide (30, 60, and 120 mg/kg) shows a decrease in T helper 17 cell cytokines such as interleukin-2 (IL-2) and an increase in regulatory T-cell cytokines in mesenteric lymph node lymphocytes, like interleukin-4 (IL-4) and transforming growth factor beta 1 (TGF-beta 1).[2][11] Another study revealed that geniposide's effect was probably enhanced by immunoregulation in immunologic tissues, such as gut-associated lymphoid tissue (GALT). When regulating, the mesenteric lymph node triggers the amelioration of the JNK-mitogen-activated protein kinases (MAPKs) and p38 mitogen-activated protein kinases (p38MAPKs) signaling cascades. The same pathway was observed in peripheral blood lymphocytes.[12]
Antidiabetic activity
Geniposide has been reported as having a hypoglycemic effect, which could be mediated by hepatic glucose-metabolizing enzymes, such as hepatic glycogen phosphorylase (GP) and glucose-6-phosphatase (G6Pase).[13] GP and G6Pase are induced by chronic hyperglycemia. High levels of blood sugar increased their expression and activity, which lead to an increase in hepatic glucose production and unbalance the glucose metabolism.[14] A study with the high-fat diet (HFD) - streptozotocin (STZ) diabetic mouse model using geniposides doses of 200 and 400 (mg/kg) has shown a significant decrease in body weight, blood glucose, insulin and triglycerides (TG) levels. An increase in the activity of GP and G6Pas was also observed in this diabetic mouse model, but when the same geniposide doses were administered, activity decreased significantly.[13]
Pharmacokinetics
Absorption
Studies show that oral (50 mg/kg), intravenous (50 mg/kg) and intramuscular (8 mg/kg) administration of Geniposide follow a one-compartment model and nasal administration (8 mg/kg) a two-compartment model. The absolute bioavailability is higher in intramuscular administration (F = 72,69%) followed by nasal administration (F = 49,54%).[15]
Distribution
In rats, after an oral administration of geniposide (200 mg/kg) the highest tissue concentration was observed in the kidney (1.12 ± 0.37 μg/ml) with a tmax of 2h. The tissue distribution, measured in terms of AUC0→4h values, follows kidney > spleen> liver > heart > lung> brain.[16]
Metabolism
Using ultrahigh-performance liquid chromatography 17 metabolites were identified in plasma and 31 in urine. In vivo, geniposide can follow two distinct metabolic pathways. The main metabolic pathway involves the hydrolysis of the hydroxyl groups followed by a series of reactions, such as taurine, sulfate and glucuronide conjugation.[3][17]
Excretion
In humans, the majority of excretion of Geniposide is urinary.[3]
Toxicity
Hepatoxicity is a safety issue of geniposide. Several studies in rats have shown an increase in serum alanine aminotransferase and aspartate aminotransferase activities (oral administration of 320 mg/kg body weight).[18] A 2012 study linked geniposide hepatoxicity with oxidative stress, due to a decrease of total superoxide dismutase activity and an increase of malondialdehyde concentration in rats’ livers. These results were associated only with a high dose of geniposide (greater than 574 mg/kg).[19] A repeated dosing study has shown that geniposide is safe at a dosage of 24.3 mg/kg or less.[19]
Acute nephrotoxicity was observed after an oral administration of geniposide (dose of 1.2 g/kg) on jaundice rats. Increases in serum levels of blood urea nitrogen and creatinine were detected.[20]
Long-term oral intake of Chinese herbal liquid containing geniposide may play a role in the pathogenesis of idiopathic mesenteric phlebosclerosis.[21]
References
- ↑ CID 387043706 from PubChem
- ↑ 2.0 2.1 "Diverse Pharmacological Activities and Potential Medicinal Benefits of Geniposide". Evidence-Based Complementary and Alternative Medicine 2019: 4925682. April 2019. doi:10.1155/2019/4925682. PMID 31118959.
- ↑ 3.0 3.1 3.2 "A Review on the Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Geniposide, a Natural Product". Molecules 22 (10): 1689. October 2017. doi:10.3390/molecules22101689. PMID 28994736.
- ↑ "Antioxidative Property and Molecular Mechanisms Underlying Geniposide-Mediated Therapeutic Effects in Diabetes Mellitus and Cardiovascular Disease". Oxidative Medicine and Cellular Longevity 2019: 7480512. April 2019. doi:10.1155/2019/7480512. PMID 31089416.
- ↑ "Neuroprotective effects of geniposide on Alzheimer's disease pathology". Reviews in the Neurosciences 26 (4): 371–83. 2015. doi:10.1515/revneuro-2015-0005. PMID 25879319.
- ↑ "Geniposide ameliorates learning memory deficits, reduces τ phosphorylation and decreases apoptosis via GSK3 β pathway in streptozotocin-induced Alzheimer rat model.". Brain Pathol. 24 (3): 261–269. 2014. doi:10.1111/bpa.12116. PMID 24329968.
- ↑ 7.0 7.1 "Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis". European Neuropsychopharmacology 25 (8): 1332–41. August 2015. doi:10.1016/j.euroneuro.2015.04.009. PMID 25914157.
- ↑ "Antidepressants and hypothalamic-pituitary-adrenocortical regulation". Endocrine Reviews 17 (2): 187–205. April 1996. doi:10.1210/edrv-17-2-187. PMID 8706631.
- ↑ "Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary hippocampal neurons". Biological & Pharmaceutical Bulletin 33 (11): 1841–6. 2010. doi:10.1248/bpb.33.1841. PMID 21048309.
- ↑ "Geniposide attenuates cadmium‑induced oxidative stress injury via Nrf2 signaling in osteoblasts". Molecular Medicine Reports 20 (2): 1499–1508. August 2019. doi:10.3892/mmr.2019.10396. PMID 31257486.
- ↑ "Antiinflammation Effects and Mechanisms Study of Geniposide on Rats with Collagen-Induced Arthritis". Phytotherapy Research 31 (4): 631–637. April 2017. doi:10.1002/ptr.5775. PMID 28127805.
- ↑ "Effects and mechanisms of geniposide on rats with adjuvant arthritis". Immunopharmacol 20 (1): 46–53. 2014. doi:10.1016/j.intimp.2014.02.021. PMID 24583144.
- ↑ 13.0 13.1 "Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin". Acta Pharmacologica Sinica 30 (2): 202–8. February 2009. doi:10.1038/aps.2008.17. PMID 19122671.
- ↑ "Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans". The Journal of Clinical Investigation 101 (6): 1203–9. March 1998. doi:10.1172/JCI579. PMID 9502760.
- ↑ "Pharmacokinetics of geniposide through 4 routes of administration". Chinese Journal of New Drugs 19 (9): 746–749+754. January 2010.
- ↑ "Pharmacokinetics, bioavailability and tissue distribution of geniposide following intravenous and peroral administration to rats". Biopharm. Drug Dispos. 35 (2): 97–103. Nov 2013. doi:10.1002/bdd.1869. PMID 24122743.
- ↑ "Comprehensive characterization of the in vitro and in vivo metabolites of geniposide in rats using ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 46 (4): 357–68. 2015. doi:10.3109/00498254.2015.1079746. PMID 26330181.
- ↑ "Hepatotoxicity of geniposide in rats". Food and Chemical Toxicology 28 (7): 515–9. July 1990. doi:10.1016/0278-6915(90)90122-4. PMID 2210524.
- ↑ 19.0 19.1 "Potential hepatotoxicity of geniposide, the major iridoid glycoside in dried ripe fruits of Gardenia jasminoides (Zhi-zi)". Natural Product Research 27 (10): 929–33. Mar 2012. doi:10.1080/14786419.2012.673604. PMID 22456001.
- ↑ "基于黄疸模型大鼠的栀子苷急性肝肾毒性研究" (in zh). Chinese Journal of Experimental Traditional Medical Formulae 21 (4): 174–178. 2015. doi:10.13422/j.cnki.syfjx.2015040174.
- ↑ "Idiopathic mesenteric phlebosclerosis associated with long-term oral intake of geniposide". World Journal of Gastroenterology 27 (22): 3097–3108. June 2021. doi:10.3748/wjg.v27.i22.3097. PMID 34168411.
 |
