Chemistry:Glutaric acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentanedioic acid | |
| Other names
Glutaric acid
Propane-1,3-dicarboxylic acid 1,3-Propanedicarboxylic acid Pentanedioic acid n-Pyrotartaric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H8O4 | |
| Molar mass | 132.12 g/mol |
| Melting point | 95 to 98 °C (203 to 208 °F; 368 to 371 K) |
| Boiling point | 200 °C (392 °F; 473 K) /20 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
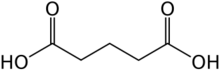
Glutaric acid is the organic compound with the formula C3H6(COOH)2. Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50% (w/w). [citation needed]
Biochemistry
Glutaric acid is naturally produced in the body during the metabolism of some amino acids, including lysine and tryptophan. Defects in this metabolic pathway can lead to a disorder called glutaric aciduria, where toxic byproducts build up and can cause severe encephalopathy.
Production
Glutaric acid can be prepared by the ring-opening of butyrolactone with potassium cyanide to give the potassium salt of the carboxylate-nitrile that is hydrolyzed to the diacid.[1] Alternatively hydrolysis, followed by oxidation of dihydropyran gives glutaric acid. It can also be prepared from reacting 1,3-dibromopropane with sodium or potassium cyanide to obtain the dinitrile, followed by hydrolysis. Using periodate, it is obtained from oxidation of 1,3-cyclohexanedione, which proceeds with decarboxylation.[2]
Uses
- 1,5-Pentanediol, a common plasticizer and precursor to polyesters is manufactured by hydrogenation of glutaric acid and its derivatives.[3]
- Glutaric acid itself has been used in the production of polymers such as polyester polyols, polyamides. The odd number of carbon atoms (i.e. 5) is useful in decreasing polymer elasticity.[4]
- Pyrogallol can be produced from glutaric diester.[5]
Safety
Glutaric acid may cause irritation to the skin and eyes.[6] Acute hazards include the fact that this compound may be harmful by ingestion, inhalation or skin absorption.[6]
References
- ↑ G. Paris, L. Berlinguet, R. Gaudry, J. English, Jr. and J. E. Dayan (1957). "Glutaric Acid and Glutaramide". Organic Syntheses: 47. doi:10.15227/orgsyn.037.0047.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1736, ISBN 978-0-471-72091-1, https://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ↑ Peter Werle and Marcus Morawietz "Alcohols, Polyhydric" in Ullmann's Encyclopedia of Industrial Chemistry: 2002, Wiley-VCH: Weinheim. DOI 10.1002/14356007.a01_305
- ↑ "Glutaric acid, Pentanedioic acid, 99%" (in en-us). http://www.chemkits.eu/carboxylic-acids/232-glutaric-acid-pentanedioic-acid-110-94-1.html.
- ↑ Shipchandler, Mohammed T., "Method of synthesis of pyrogallol", US patent 4046817, published 1977-09-06
- ↑ 6.0 6.1 Glutaric acid, cameochemicals.com
External links
 |

