Chemistry:Heneicosane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Henicosane | |
| Other names
n-Heneicosane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1748500 | |
| ChEBI | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H44 | |
| Molar mass | 296.583 g·mol−1 |
| Appearance | Waxy solid |
| Density | 0.7919 g mL−1 |
| Melting point | 40.5 °C (104.9 °F; 313.6 K) |
| Boiling point | 356.10 °C; 672.98 °F; 629.25 K |
| 2.9×10−11 g/L | |
| log P | 10.65 |
| Vapor pressure | 8.73X10-5 mm Hg |
Henry's law
constant (kH) |
120 atm•m3/mole |
Refractive index (nD)
|
1.4441 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 113 °C (235 °F; 386 K) |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
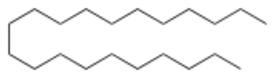
Heneicosane is the organic compound with the formula CH3(CH2)19CH3. It is the straight chain, saturated C21 hydrocarbon. It is a white wax.
Natural occurrence
Heneicosane is used as a pheromone by the queen or king termites in the species Reticulitermes flavipes.[1] It also attracts mosquitoes in the genus Aedes and can be used in mosquito baits.[2] This works in nature as the hydrocarbon is produced in the skin of the larva. A 1:100000 fraction in water is the most attractive, but if the concentration is 1:1000 then mosquitoes are repelled instead.[3] Heneicosane is one of the major components of the safflower flower essential oil (Carthamus tinctorius).[4] All parts of the plant Periploca laevigata contain heneicosane.[5] Rosa damascena flower essential oil contains 5% heneicosane.[6] Sambucus nigra contains 2.3%.
References
- ↑ "Termite queen, king recognition pheromone identified". 19 March 2018. https://phys.org/news/2018-03-termite-queen-king-recognition-pheromone.html.
- ↑ Kumar, P; Lomash, V; Jatav, PC; Kumar, A; Pant, SC (January 2016). "Prenatal developmental toxicity study of n-heneicosane in Wistar rats.". Toxicology and Industrial Health 32 (1): 118–25. doi:10.1177/0748233713498438. PMID 24060842. Bibcode: 2016ToxIH..32..118K.
- ↑ Seenivasagan, T; Sharma, KR; Sekhar, K; Ganesan, K; Prakash, S; Vijayaraghavan, R (March 2009). "Electroantennogram, flight orientation, and oviposition responses of Aedes aegypti to the oviposition pheromone n-heneicosane.". Parasitology Research 104 (4): 827–33. doi:10.1007/s00436-008-1263-2. PMID 19018567.
- ↑ Asgarpanah, J; Kazemivash, N (February 2013). "Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L.". Chinese Journal of Integrative Medicine 19 (2): 153–9. doi:10.1007/s11655-013-1354-5. PMID 23371463.
- ↑ Zito, P; Sajeva, M; Bruno, M; Rosselli, S; Maggio, A; Senatore, F (2013). "Essential oils composition of Periploca laevigata Aiton subsp. angustifolia (Labill.) Markgraf (Apocynaceae-Periplocoideae).". Natural Product Research 27 (3): 255–65. doi:10.1080/14786419.2012.671319. PMID 22439883.
- ↑ Sadraei, H; Asghari, G; Emami, S (January 2013). "Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction.". Research in Pharmaceutical Sciences 8 (1): 17–23. PMID 24459472.
 |

