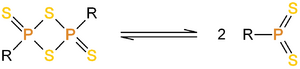
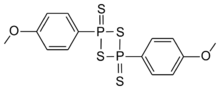
Chemistry:Lawesson's reagent
| |
| |
| Names | |
|---|---|
| IUPAC name
2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide
| |
| Preferred IUPAC name
2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-dithione | |
| Other names
Lawesson reagent; LR
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H14O2P2S4 | |
| Molar mass | 404.45 g·mol−1 |
| Appearance | Slightly yellow powder |
| Melting point | 228–231 °C (442–448 °F; 501–504 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H261, H302, H312, H332 | |
| P231+232, P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P370+378, P402+404, P501 | |
| Related compounds | |
Related thiation agents
|
Hydrogen sulfide, Phosphorus pentasulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lawesson's reagent (LR) is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.[1]
Preparation
Lawesson's reagent is commercially available. It can also be conveniently prepared in the laboratory by heating a mixture of anisole with phosphorus pentasulfide until the mixture is clear and no more hydrogen sulfide is formed,[2] then recrystallized from toluene or xylene.
Samples give a strong odor of hydrogen sulfide owing to partial hydrolysis. One common and effective method of destroying the foul smelling residues is to use an excess of sodium hypochlorite (chlorine bleach).
Mechanism of action
Lawesson's reagent has a four membered ring of alternating sulfur and phosphorus atoms. The central phosphorus/sulfur four-membered ring dissociates to form two reactive dithiophosphine ylides (R-PS2). Much of the chemistry of Lawessons's reagent is in fact the chemistry of this reactive intermediate.
In general, the more electron rich a carbonyl is, the faster the carbonyl group will be converted into the corresponding thiocarbonyl by Lawesson's reagent.
Applications
The chemistry of Lawesson's reagent and related substances has been reviewed by several times.[3][4][5][6] The main use of Lawesson's reagent is the thionation of carbonyl compounds. For instance, Lawesson's reagent will convert a carbonyl into a thiocarbonyl.[7] Additionally, Lawesson's reagent has been used to thionate enones, esters,[8] lactones,[9] amides, lactams,[10] and quinones.
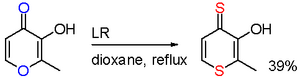
In one study, reaction of maltol with LR results in a selective oxygen replacement in two positions.[11]
A combination of silver perchlorate and Lawesson's reagent is able to act as an oxophilic Lewis acid with the ability to catalyze the Diels–Alder reaction of dienes with α,β-unsaturated aldehydes.
in some cases, alcohols may be converted to thiols by treatment with Lawesson's reagent.[12]
Lawesson's reagent reacts with sulfoxides to form thioethers.[5]
See also
References
- ↑ Lecher, H. Z.; Greenwood, R. A.; Whitehouse, K. C.; Chao, T. H. (1956). "The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide". J. Am. Chem. Soc. 78 (19): 5018. doi:10.1021/ja01600a058.
- ↑ Thomsen, I.; Clausen, K.; Scheibye, S.; Lawesson, S.-O. (1984). "Thiation with 2,4-Bis(4-methoxyphenyl)-1,3,2,4-Dithiadiphosphetane 2,4-disulfide: N-Methylthiopyrrolidone". Organic Syntheses 62: 158. doi:10.15227/orgsyn.062.0158.
- ↑ Cherkasov, R. A.; Kutyrev, G. A.; Pudovik, A. N. (1985). "Tetrahedron report number 186 Organothiophosphorus reagents in organic synthesis". Tetrahedron 41 (13): 2567. doi:10.1016/S0040-4020(01)96363-X.
- ↑ Foreman, M.S.; Woollins, J.D. (2000). "Organo-P–S and P–Se heterocycles". J. Chem. Soc., Dalton Trans. (10): 1533–1543. doi:10.1039/b000620n.
- ↑ 5.0 5.1 Martin Jesberger; Thomas P. Davis; Leonie Barner (2003). "Applications of Lawesson's Reagent in Organic and Organometallic Syntheses". Synthesis 2003 (13): 1929–1958. doi:10.1055/s-2003-41447. https://github.com/JeremyMGibson/verbose-garbanzo/raw/master/97507/600350.
- ↑ Cava, M. P.; Levinson, M. I. (1985). "Thionation reactions of Lawesson's reagents". Tetrahedron 41 (22): 5061–5087. doi:10.1016/S0040-4020(01)96753-5.
- ↑ Pedersen, B. S.; Scheibye, S.; Nilsson, N. H.; Lawesson, S.-O. (1978). "Studies on organophosphorus compounds XX. syntheses of thioketones". Bull. Soc. Chim. Belg. 87 (3): 223–228. doi:10.1002/bscb.19780870310.
- ↑ Jones, B. A.; Bradshaw, J. S. (1984). "Synthesis and reduction of thiocarboxylic O-esters". Chem. Rev. 84 (84): 17. doi:10.1021/cr00059a002.
- ↑ Scheibye, S.; Kristensen, J.; Lawesson, S.-O. (1979). "Studies on organophosphorus compounds XXVII. Synthesis of thiono-, thiolo- and dithiolactones". Tetrahedron 35 (11): 1339–1343. doi:10.1016/0040-4020(79)85027-9.
- ↑ Shabana, R.; Scheibye, S.; Clausen, K.; Olesen, S. O.; Lawesson, S.-O. (1980). "Studies on organophosphorus compounds XXXI. Synthesis of thiolactams and thioimides". Nouveau Journal de Chimie 1980 (4): 47.
- ↑ Brayton, D.; Jacobsen, F. E.; Cohen, S. M.; Farmer, P. J. (2006). "A novel heterocyclic atom exchange reaction with Lawesson's reagent: a one-pot synthesis of dithiomaltol". Chemical Communications 2006 (2): 206–208. doi:10.1039/b511966a. PMID 16372107.
- ↑ Nishio, Takehiko (1989). "A novel transformation of alcohols to thiols". Journal of the Chemical Society, Chemical Communications 1989 (4): 205–206. doi:10.1039/C39890000205.
External links
- "Lawesson's Reagent". Organic Chemistry Portal. https://www.organic-chemistry.org/namedreactions/lawessons-reagent.shtm. Retrieved 2007-10-16.
 |