Chemistry:Magnesium iodide
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Magnesium iodide
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| |||
| Molar mass |
| ||
| Appearance | white crystalline solid | ||
| Odor | odorless | ||
| Density |
| ||
| Melting point | 637 °C (1,179 °F; 910 K) (anhydrous, decomposes) 41 °C (octahydrate, decomposes) | ||
| |||
| Solubility | soluble in ether, alcohol and ammonia | ||
| −111.0·10−6 cm3/mol | |||
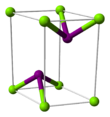
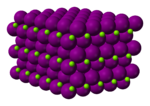
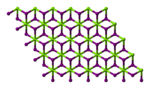
| Structure | |||
| |||
| Thermochemistry | |||
Heat capacity (C)
|
74 J/(mol·K) | ||
Std molar
entropy (S |
134 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−364 kJ/mol | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H315, H319 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
|||
Other cations
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Magnesium iodide is an inorganic compound with the chemical formula MgI
2. It forms various hydrates MgI
2 · xH
2O. Magnesium iodide is a salt of magnesium and hydrogen iodide. These salts are typical ionic halides, being highly soluble in water.
Uses
Magnesium iodide has few commercial uses, but can be used to prepare compounds for organic synthesis.
Preparation
Magnesium iodide can be prepared from magnesium oxide, magnesium hydroxide, and magnesium carbonate by treatment with hydroiodic acid:[3]
Reactions
Magnesium iodide is stable at high heat under a hydrogen atmosphere, but decomposes in air at normal temperatures, turning brown from the release of elemental iodine. When heated in air, it decomposes completely to magnesium oxide.[4]
Another method to prepare MgI
2 is mixing powdered elemental iodine and magnesium metal. In order to obtain anhydrous MgI
2, the reaction should be conducted in a strictly anhydrous atmosphere; dry-diethyl ether can be used as a solvent.
Usage of magnesium iodide in the Baylis-Hillman reaction tends to give (Z)-vinyl compounds.[5]
Demethylation of certain aromatic methyl ethers can be afforded using magnesium iodide in diethyl ether.[6]
Hydrates
Two hydrates are known, the octahydrate and the nonahydrate, both verified by X-ray crystallography These hydrates feature [Mg(H2O)6]2+ ions.[7]
References
- ↑ Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, pp. 240, ISBN 0-8493-8671-3, https://books.google.com/books?id=0fT4wfhF1AsC&q=%22magnesium+iodide%22&pg=PA240, retrieved 2007-12-09
- ↑ Magnesium Iodide MSDS at AlfaAesar[yes|permanent dead link|dead link}}]
- ↑ Patnaik, Pradyot (2003), Handbook of Inorganic Chemicals, McGraw-Hill Professional, pp. 527–528, ISBN 0-07-049439-8, https://books.google.com/books?id=Xqj-TTzkvTEC&q=%22magnesium+iodide%22&pg=RA1-PA527, retrieved 2007-12-09
- ↑ Wilsmore, N. T. M. (1891). "Note on Magnesium Iodide". in James Hector. Sydney: The Association. pp. 116. https://books.google.com/books?id=ktw4AAAAMAAJ&q=%22magnesium+iodide%22&pg=PA116. Retrieved 2007-12-09.
- ↑ Tietze, Lutz-Friedjan; Brasche, Gordon; Gericke, Kersten (2006), "Domino Reactions in Organic Synthesis", Chemical Reviews (Wiley-VCH) 96 (1): 115–136, doi:10.1021/cr950027e, ISBN 3-527-29060-5, PMID 11848746, https://books.google.com/books?id=qijhLyZ6SokC&q=%22magnesium+iodide%22+reactions&pg=PA59, retrieved 2007-12-09
- ↑ Yamaguchi, Seiji; Nedachi, Masahiro; Yokoyama, Hajime; Hirai, Yoshiro (October 1999). "Regioselective demethylation of 2,6-dimethoxybenzaldehydes with magnesium iodide etherate". Tetrahedron Letters 40 (41): 7363–7365. doi:10.1016/S0040-4039(99)01411-2.
- ↑ Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2013). "Crystal Structures of Hydrates of Simple Inorganic Salts. I. Water-Rich Magnesium Halide Hydrates MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O". Acta Crystallographica Section C Crystal Structure Communications 69 (11): 1292–1300. doi:10.1107/S0108270113028138. PMID 24192174.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |