Chemistry:Magnesium bromide
| |
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| |
| Molar mass | 184.113 g/mol (anhydrous) 292.204 g/mol (hexahydrate) |
| Appearance | white hygroscopic hexagonal crystals (anhydrous) colorless monoclinic crystals (hexahydrate) |
| Density | 3.72 g/cm3 (anhydrous) 2.07 g/cm3 (hexahydrate) |
| Melting point | 711 °C (1,312 °F; 984 K) 172.4 °C, decomposes (hexahydrate) |
| Boiling point | 1,250 °C (2,280 °F; 1,520 K) |
| 102 g/(100 mL) (anhydrous) 316 g/(100 mL) (0 °C, hexahydrate) | |
| Solubility | ethanol: 6.9 g/(100 mL) methanol: 21.8 g/(100 mL) |
| −72.0·10−6 cm3/mol | |
| Structure | |
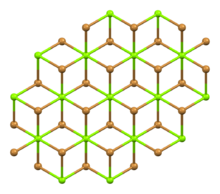
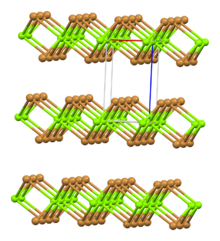
| Rhombohedral, hP3 | |
| P-3m1, No. 164 | |
| octahedral | |
| Thermochemistry | |
Heat capacity (C)
|
70 J/(mol·K) |
Std molar
entropy (S |
117.2 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−524.3 kJ/mol |
| Hazards | |
| Safety data sheet | External SDS |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium bromide are inorganic compounds with the chemical formula MgBr
2(H
2O)
x, where x can range from 0 to 9. They are all white deliquescent solids. Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.[2][3]
Synthesis
Magnesium bromide can be synthesized by treating magnesium oxide (and related basic salts) with hydrobromic acid.[3] It can also be made by reacting magnesium carbonate and hydrobromic acids, and collecting the solid left after evaporation.[2]
As suggested by its easy conversion to various hydrates, anhydrous MgBr
2 is a Lewis acid. In the coordination polymer with the formula MgBr2(dioxane)2, Mg2+ adopts an octahedral geometry.[4]
Uses and reactions
Magnesium bromide is used as a Lewis acid catalyst in some organic synthesis, e.g., in aldol reaction.[5]
Magnesium bromide also has been used as a tranquilizer[2] and as an anticonvulsant for treatment of nervous disorders.[6]
Magnesium bromide modifies the catalytic properties of palladium on charcoal.[7]
Magnesium bromide hexahydrate has properties as a flame retardant.[8]
Treatment of magnesium bromide with chlorine gives magnesium chloride. This reaction is employed in the production of magnesium chloride from brines.[9]
Structure
Two hydrates are known, the hexahydrate and the nonahydrate. Several reports claim a decahydrate, but X-ray crystallography confirmed that it is a nonahydrate. The hydrates feature [Mg(H2O)6]2+ ions.[10]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–67. ISBN 0-8493-0594-2.
- ↑ 2.0 2.1 2.2 Gruyter, W. Concise Encyclopedia Chemistry, Walter de Gruyter & Company: Berlin, 1993; 612
- ↑ 3.0 3.1 Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons Inc.:New York, 2007; 777
- ↑ Fischer, Reinald; Görls, Helmar; Meisinger, Philippe R.; Suxdorf, Regina; Westerhausen, Matthias (2019). "Structure–Solubility Relationship of 1,4-Dioxane Complexes of Di(hydrocarbyl)magnesium". Chemistry – A European Journal 25 (55): 12830–12841. doi:10.1002/chem.201903120. PMID 31328293.
- ↑ Evans, David A.; Tedrow, Jason S.; Shaw, Jared T.; Downey, C. Wade (2002). "Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones". Journal of the American Chemical Society 124 (3): 392–393. doi:10.1021/ja0119548. PMID 11792206.
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ Bouzide, Abderrahim (2002). "Magnesium Bromide Mediated Highly Diastereoselective Heterogeneous Hydrogenation of Olefins". Organic Letters 4 (8): 1347–50. doi:10.1021/ol020032m. PMID 11950359.
- ↑ Mostashari, S. M.; Fayyaz, F. (2008). "XRD characterization of the ashes from a burned cellulosic fabric impregnated with magnesium bromide hexahydrate as flame-retardant". Journal of Thermal Analysis and Calorimetry 92 (3): 845. doi:10.1007/s10973-007-8928-4.
- ↑ Seeger, Margarete; Otto, Walter; Flick, Wilhelm; Bickelhaupt, Friedrich; Akkerman, Otto S. (2000). "Magnesium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_595. ISBN 3-527-30673-0.
- ↑ Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2013). "Crystal Structures of Hydrates of Simple Inorganic Salts. I. Water-Rich Magnesium Halide Hydrates MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O". Acta Crystallographica Section C Crystal Structure Communications 69 (11): 1292–1300. doi:10.1107/S0108270113028138. PMID 24192174.
 |

