Chemistry:Magnesium chloride

| |
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 9305 | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| |
| |
| Properties | |
| MgCl 2 | |
| Molar mass | 95.211 g/mol (anhydrous) 203.31 g/mol (hexahydrate) |
| Appearance | white or colourless crystalline solid |
| Density | 2.32 g/cm3 (anhydrous) 1.569 g/cm3 (hexahydrate) |
| Melting point | 714 °C (1,317 °F; 987 K) anhydrous 117 °C (243 °F; 390 K) hexahydrate on rapid heating; slow heating leads to decomposition from 300 °C (572 °F; 573 K) |
| Boiling point | 1,412 °C (2,574 °F; 1,685 K) |
| |
| Solubility | slightly soluble in acetone, pyridine |
| Solubility in ethanol | 7.4 g/(100 mL) (30 °C) |
| −47.4·10−6 cm3/mol | |
Refractive index (nD)
|
1.675 (anhydrous) 1.569 (hexahydrate) |
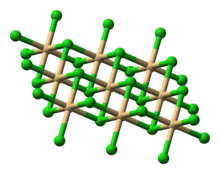
| Structure | |
| CdCl 2 | |
| (octahedral, 6-coordinate) | |
| Thermochemistry | |
Heat capacity (C)
|
71.09 J/(mol·K) |
Std molar
entropy (S |
89.88 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−641.1 kJ/mol |
Gibbs free energy (ΔfG˚)
|
−591.6 kJ/mol |
| Pharmacology | |
| 1=ATC code }} | A12CC01 (WHO) B05XA11 (WHO) |
| Hazards[1] | |
| Main hazards | Irritant |
| Safety data sheet | ICSC 0764 |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319, H335 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2800 mg/kg (oral, rat) |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium chloride is an inorganic compound with the formula MgCl
2. It forms hydrates MgCl
2 · nH
2O, where n can range from 1 to 12. These salts are colorless or white solids that are highly soluble in water. These compounds and their solutions, both of which occur in nature, have a variety of practical uses. Anhydrous magnesium chloride is the principal precursor to magnesium metal, which is produced on a large scale. Hydrated magnesium chloride is the form most readily available.[2]
Production
Magnesium chloride can be extracted from brine or sea water. In North America and South America, it is obtained primarily from Great Salt Lake brine. In the Jordan Valley, it is obtained from the Dead Sea. The mineral bischofite (MgCl
2 · 6H2O) is extracted (by solution mining) out of ancient seabeds, for example, the Zechstein seabed in northwest Europe. Some deposits result from high content of magnesium chloride in the primordial ocean.[3] Some magnesium chloride is made from evaporation of seawater.
In the Dow process, magnesium chloride is regenerated from magnesium hydroxide using hydrochloric acid:
It can also be prepared from magnesium carbonate by a similar reaction.
Structure
MgCl
2 crystallizes in the cadmium chloride CdCl
2 motif, therefore it loses water upon heating: n = 12 (−16.4 °C), 8 (−3.4 °C), 6 (116.7 °C), 4 (181 °C), 2 (about 300 °C).[4] In the hexahydrate, the Mg2+ is also octahedral, being coordinated to six water ligands.[5] The octahydrate and the dodecahydrate can be crystallized from water below 298K. As verified by X-ray crystallography, these "higher" hydrates also feature [Mg(H2O)6]2+ ions.[6] A decahydrate has also been crystallized.[7]
Preparation, general properties
Anhydrous MgCl
2 is produced industrially by heating the complex salt named hexamminemagnesium dichloride [Mg(NH
3)
6]2+(Cl−
)
2.[2] The thermal dehydration of the hydrates MgCl
2 · nH
2O (n = 6, 12) does not occur straightforwardly.[8]
As suggested by the existence of hydrates, anhydrous MgCl
2 is a Lewis acid, although a weak one. One derivative is tetraethylammonium tetrachloromagnesate [N(CH
2CH
3)
4]
2[MgCl
4]. The adduct MgCl
2(TMEDA) is another.[9] In the coordination polymer with the formula MgCl
2(dioxane)
2, Mg adopts an octahedral geometry.[10] The Lewis acidity of magnesium chloride is reflected in its deliquescence, meaning that it attracts moisture from the air to the extent that the solid turns into a liquid.
Applications
Precursor to metallic magnesium
Anhydrous MgCl
2 is the main precursor to metallic magnesium. The reduction of Mg2+ into metallic Mg is performed by electrolysis in molten salt.[2][11] As it is also the case for aluminium, an electrolysis in aqueous solution is not possible as the produced metallic magnesium would immediately react with water, or in other words that the water H+
would be reduced into gaseous H
2 before Mg reduction could occur. So, the direct electrolysis of molten MgCl
2 in the absence of water is required because the reduction potential to obtain Mg is lower than the stability domain of water on an Eh–pH diagram (Pourbaix diagram).
- MgCl
2 → Mg + Cl
2
The production of metallic magnesium at the cathode (reduction reaction) is accompanied by the oxidation of the chloride anions at the anode with release of gaseous chlorine. This process is developed at a large industrial scale.
Dust and erosion control
Magnesium chloride is one of many substances used for dust control, soil stabilization, and wind erosion mitigation.[12] When magnesium chloride is applied to roads and bare soil areas, both positive and negative performance issues occur which are related to many application factors.[13]
Catalysis
Ziegler-Natta catalysts, used commercially to produce polyolefins, often contain MgCl
2 as a catalyst support.[14] The introduction of MgCl
2 supports increases the activity of traditional catalysts and allowed the development of highly stereospecific catalysts for the production of polypropylene.[15]
Magnesium chloride is also a Lewis acid catalyst in aldol reactions.[16]
Ice control

Magnesium chloride is used for low-temperature de-icing of highways, sidewalks, and parking lots. When highways have dangerous ice buildup, road maintainers apply magnesium chloride to deter ice from bonding to the pavement, allowing snow plows to clear treated roads more efficiently.
For the purpose of preventing ice from forming on pavement, magnesium chloride is applied in three ways: anti-icing, which involves spreading it on roads to prevent snow from sticking and forming; prewetting, which means a liquid formulation of magnesium chloride is sprayed directly onto salt as it is being spread onto roadway pavement, wetting the salt so that it sticks to the road; and pretreating, when magnesium chloride and salt are mixed together before they are loaded onto trucks and spread onto paved roads. Calcium chloride damages concrete twice as fast as magnesium chloride.[17] The amount of magnesium chloride is supposed to be controlled when it is used for de-icing as it may cause pollution to the environment.[18]
Nutrition and medicine
Magnesium chloride is used in nutraceutical and pharmaceutical preparations. The hexahydrate is sometimes advertised as "magnesium oil". Magnesium Chloride is also an electrolyte.
Cuisine
Magnesium chloride (E511[19]) is an important coagulant used in the preparation of tofu from soy milk.
In Japan it is sold as nigari (にがり, derived from the Japanese word for "bitter"), a white powder produced from seawater after the sodium chloride has been removed, and the water evaporated. In China, it is called lushui (卤水).
Nigari or Lushui is, in fact, natural magnesium chloride, meaning that it is not completely refined (it contains up to 5% magnesium sulfate and various minerals). The crystals originate from lakes in the Chinese province of Qinghai, to be then reworked in Japan.
Gardening and horticulture
Because magnesium is a mobile nutrient, magnesium chloride can be effectively used as a substitute for magnesium sulfate (Epsom salt) to help correct magnesium deficiency in plants via foliar feeding. The recommended dose of magnesium chloride is smaller than the recommended dose of magnesium sulfate (20 g/L).[20] This is due primarily to the chlorine present in magnesium chloride, which can easily reach toxic levels if over-applied or applied too often.[21]
It has been found that higher concentrations of magnesium in tomato and some pepper plants can make them more susceptible to disease caused by infection of the bacterium Xanthomonas campestris, since magnesium is essential for bacterial growth.[22]
Wastewater treatment
It is used to supply the magnesium necessary to precipitate phosphorus in the form of struvite from agricultural waste[23] as well as human urine.
Occurrence
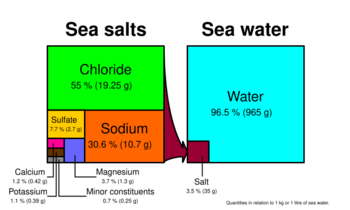
Magnesium concentrations in natural seawater are between 1250 and 1350 mg/L, around 3.7% of the total seawater mineral content. Dead Sea minerals contain a significantly higher magnesium chloride ratio, 50.8%. Carbonates and calcium[clarification needed] are essential for all growth of corals, coralline algae, clams, and invertebrates. Magnesium can be depleted by mangrove plants and the use of excessive limewater or by going beyond natural calcium, alkalinity, and pH values.[24] The most common mineral form of magnesium chloride is its hexahydrate, bischofite.[25][26] Anhydrous compound occurs very rarely, as chloromagnesite.[26] Magnesium chloride-hydroxides, korshunovskite and nepskoeite, are also very rare.[27][28][26]
Toxicology
Magnesium ions are bitter-tasting, and magnesium chloride solutions are bitter in varying degrees, depending on the concentration.
Magnesium toxicity from magnesium salts is rare in healthy individuals with a normal diet, because excess magnesium is readily excreted in urine by the kidneys. A few cases of oral magnesium toxicity have been described in persons with normal renal function ingesting large amounts of magnesium salts, but it is rare. If a large amount of magnesium chloride is eaten, it will have effects similar to magnesium sulfate, causing diarrhea, although the sulfate also contributes to the laxative effect in magnesium sulfate, so the effect from the chloride is not as severe.
Plant toxicity
Chloride (Cl−
) and magnesium (Mg2+) are both essential nutrients important for normal plant growth. Too much of either nutrient may harm a plant, although foliar chloride concentrations are more strongly related with foliar damage than magnesium. High concentrations of MgCl
2 ions in the soil may be toxic or change water relationships such that the plant cannot easily accumulate water and nutrients. Once inside the plant, chloride moves through the water-conducting system and accumulates at the margins of leaves or needles, where dieback occurs first. Leaves are weakened or killed, which can lead to the death of the tree.[29]
See also
Notes and references
- Notes
- ↑ "Summary of Classification and Labelling". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/93509.
- ↑ 2.0 2.1 2.2 Margarete Seeger; Walter Otto; Wilhelm Flick; Friedrich Bickelhaupt; Otto S. Akkerman. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_595.pub2.
- ↑ Hisahiro Ueda and Takazo Shibuya (2021). "Composition of the Primordial Ocean Just after Its Formation: Constraints from the Reactions between the Primitive Crust and a Strongly Acidic, CO2-Rich Fluid at Elevated Temperatures and Pressures". Minerals (Minerals 2021, 11(4), p. 389) 11 (4): 389. doi:10.3390/min11040389. Bibcode: 2021Mine...11..389U.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2013). "Crystal Structures of Hydrates of Simple Inorganic Salts. I. Water-Rich Magnesium Halide Hydrates MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O". Acta Crystallographica Section C Crystal Structure Communications 69 (11): 1292–1300. doi:10.1107/S0108270113028138. PMID 24192174.
- ↑ Komatsu, Kazuki; Shinozaki, Ayako; Machida, Shinichi; Matsubayashi, Takuto; Watanabe, Mao; Kagi, Hiroyuki; Sano-Furukawa, Asami; Hattori, Takanori (2015). "Crystal structure of magnesium dichloride decahydrate determined by X-ray and neutron diffraction under high pressure". Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials 71 (Pt 1): 74–80. doi:10.1107/S205252061500027X. PMID 25643718.
- ↑ See notes in Rieke, R. D.; Bales, S. E.; Hudnall, P. M.; Burns, T. P.; Poindexter, G. S. "Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornane Acid", Organic Syntheses, Collected Volume 6, p. 845 (1988). "Archived copy". http://www.orgsyn.org/orgsyn/pdfs/CV6P0845.pdf.
- ↑ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, 1984.
- ↑ Fischer, Reinald; Görls, Helmar; Meisinger, Philippe R.; Suxdorf, Regina; Westerhausen, Matthias (2019). "Structure–Solubility Relationship of 1,4-Dioxane Complexes of Di(hydrocarbyl)magnesium". Chemistry – A European Journal 25 (55): 12830–12841. doi:10.1002/chem.201903120. PMID 31328293. Bibcode: 2019ChEuJ..2512830F.
- ↑ Hill, Petrucci, McCreary, Perry, General Chemistry, 4th ed., Pearson/Prentice Hall, Upper Saddle River, New Jersey, USA.
- ↑ "Dust Palliative Selection and Application Guide". Fs.fed.us. http://www.fs.fed.us/eng/pubs/html/99771207/99771207.html#EI.
- ↑ "FSE Documents". https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1043546.pdf.
- ↑ Dennis B. Malpass (2010). "Commercially Available Metal Alkyls and Their Use in Polyolefin Catalysts". in Ray Hoff. Handbook of Transition Metal Polymerization Catalysts. John Wiley & Sons, Inc.. pp. 1–28. doi:10.1002/9780470504437.ch1. ISBN 9780470504437.
- ↑ Norio Kashiwa (2004). "The Discovery and Progress of MgCl2-Supported TiCl4 Catalysts". Journal of Polymer Science A 42 (1): 1–8. doi:10.1002/pola.10962. Bibcode: 2004JPoSA..42....1K.
- ↑ Evans, David A.; Tedrow, Jason S.; Shaw, Jared T.; Downey, C. Wade (2002). "Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones". Journal of the American Chemical Society 124 (3): 392–393. doi:10.1021/ja0119548. PMID 11792206. Bibcode: 2002JAChS.124..392E.
- ↑ Jain, J., Olek, J., Janusz, A., and Jozwiak-Niedzwiedzka, D., "Effects of Deicing Salt Solutions on Physical Properties of Pavement Concretes", Transportation Research Record: Journal of the Transportation Research Board, No. 2290, Transportation Research Board of the National Academies, Washington, D.C., 2012, pp. 69-75. doi:10.3141/2290-09.
- ↑ Dai, H.L.; Zhang, K.L.; Xu, X.L.; Yu, H.Y. (2012). "Evaluation on the Effects of Deicing Chemicals on Soil and Water Environment" (in en). Procedia Environmental Sciences 13: 2122–2130. doi:10.1016/j.proenv.2012.01.201. Bibcode: 2012PrEnS..13.2122D.
- ↑ Food Standard Agency. "Current EU approved additives and their E Numbers". http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist.
- ↑ "Comparison of Magnesium Sulfate and THIS Mg Chelate Foliar Sprays". Canadian Journal of Plant Science. January 1985. doi:10.4141/cjps85-018.
- ↑ "Magnesium Chloride Toxicity in Trees". Ext.colostate.edu. http://www.ext.colostate.edu/pubs/garden/07425.html.
- ↑ "Effect of Foliar and Soil Magnesium Application on Bacterial Leaf Spot of Peppers". http://www.apsnet.org/publications/plantdisease/backissues/Documents/1983Articles/PlantDisease67n06_623.pdf.
- ↑ BURNS, R.T. (15 January 2001). "Laboratory and In-Situ Reductions of Soluble Phosphorus in Swine Waste Slurries". Environmental Technology 22 (11): 1273–1278. doi:10.1080/09593332208618190. PMID 11804348. Bibcode: 2001EnvTe..22.1273B. http://www.stormwater.ucf.edu/chemicaltreatment/documents/Burns%20et%20al.,%202001.pdf. Retrieved 30 December 2023.
- ↑ "Aquarium Chemistry: Magnesium In Reef Aquaria — Advanced Aquarist | Aquarist Magazine and Blog". Advancedaquarist.com. 2003-10-15. http://www.advancedaquarist.com/issues/oct2003/chem.htm.
- ↑ "Bischofite: Mineral information, data and localities". https://www.mindat.org/min-681.html.
- ↑ 26.0 26.1 26.2 "List of Minerals". 21 March 2011. https://www.ima-mineralogy.org/Minlist.htm.
- ↑ "Korshunovskite: Mineral information, data and localities". https://www.mindat.org/min-2256.html.
- ↑ "Nepskoeite: Mineral information, data and localities". https://www.mindat.org/min-7189.html.
- ↑ "Publications – ExtensionExtension". Ext.colostate.edu. http://www.ext.colostate.edu/pubs/garden/07425.html.
- References
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
External links
 |

