Chemistry:Magnesium citrate
| |

| |
| Names | |
|---|---|
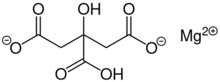
| IUPAC name
Magnesium 2-hydroxypropane-1,2,3-tricarboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H6MgO7 | |
| Molar mass | 214.412 g·mol−1 |
| 20 g/100ml | |
| Pharmacology | |
| 1=ATC code }} | A06AD19 (WHO) A12CC04 (WHO), B05CB03 (WHO) |
| Related compounds | |
Related salts
|
Chemistry:Magnesium citrate (3:2) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium citrates are metal-organic compounds formed from citrate and magnesium ions. They are salts. One form is the 1:1 magnesium preparation in salt form with citric acid in a 1:1 ratio (1 magnesium atom per citrate molecule). It contains 11.33% magnesium by weight. Magnesium citrate (sensu lato) is used medicinally as a saline laxative and to empty the bowel before major surgery or a colonoscopy. It is available without a prescription, both as a generic and under various brand names. It is also used in the pill form as a magnesium dietary supplement. As a food additive, magnesium citrate is used to regulate acidity and is known as E number E345.
Structures
The structures of solid magnesium citrates have been characterized by X-ray crystallography. In the 1:1 salt, only one carboxylate of citrate is deprotonated. It has the formula Mg(H
2C
6H
5O
7)
2 The other form of magnesium citrate has the formula Mg(HC
6H
5O
7)(H
2O)
2, consisting of the citrate dianion (both carboxylic acids are deprotonated).[1] Thus, it is clear that name "magnesium citrate" is ambiguous and sometimes may refer to other salts such as trimagnesium dicitrate which has a magnesium:citrate ratio of 3:2, or monomagnesium dicitrate with a ratio of 1:2, or a mix of two or three of the salts of magnesium and citric acid.
Mechanism of action
Magnesium citrate works by attracting water through the tissues by a process known as osmosis. Once in the intestine, it can attract enough water into the intestine to induce defecation.[2][3] The additional water stimulates bowel motility. This means it can also be used to treat rectal and colon problems. Magnesium citrate functions best on an empty stomach, and should always be followed with a full (eight-ounce or 250 ml) glass of water or juice to help counteract water loss and aid in absorption. Magnesium citrate solutions generally produce bowel movement in one-half to three hours.[4]
Use and dosage
The maximum upper tolerance limit (UTL) for magnesium in supplement form for adults is 350 mg of elemental magnesium per day, according to the National Institutes of Health (NIH).[5] In addition, according to the NIH, total dietary requirements for magnesium from all sources (in other words, food and supplements) is 320–420 mg of elemental magnesium per day, though there is no UTL for dietary magnesium.
Laxative
Magnesium citrate is used as a laxative agent.[6]
Magnesium deficiency treatment
Although less common, as a magnesium supplement the citrate form is sometimes used because it is believed to be more bioavailable than other common pill forms, such as magnesium oxide.[7] But, according to one study, magnesium gluconate was found to be marginally more bioavailable than even magnesium citrate.[8]
Potassium-magnesium citrate, as a supplement in pill form, is useful for the prevention of kidney stones.[9]
Side effects
Magnesium citrate is generally not a harmful substance, but care should be taken by consulting a healthcare professional if any adverse health problems are suspected or experienced. Extreme magnesium overdose can result in serious complications such as slow heartbeat, low blood pressure, nausea, drowsiness, etc. If severe enough, an overdose can even result in coma or death.[10][11] However, a moderate overdose will be excreted through the kidneys, unless one has serious kidney problems. Rectal bleeding or failure to have a bowel movement after use could be signs of a serious condition.
See also
References
- ↑ Kaduk, James A. (2020). "Crystal structures of two magnesium citrates from powder diffraction data". Acta Crystallographica Section E 76 (10): 1611–1616. doi:10.1107/S2056989020011913. PMID 33117574. Bibcode: 2020AcCrE..76.1611K.
- ↑ Song, Ki Hwan; Suh, Wu Seok; Jeong, Jin Sik; Kim, Dong Sik; Kim, Sang Woo; Kwak, Dong Min; Hwang, Jong Seong; Kim, Hyun Jin et al. (October 2014). "Effectiveness of Sodium Picosulfate/Magnesium Citrate (PICO) for Colonoscopy Preparation". Annals of Coloproctology 30 (5): 222–227. doi:10.3393/ac.2014.30.5.222. PMID 25360429.
- ↑ Ogbru, Omudhome. "Magnesium citrate for Constipation, Uses, Side Effects, Dosage". MedicineNet. https://www.medicinenet.com/magnesium_citrate-oral/article.htm.
- ↑ "Magnesium Citrate". WebMD. http://www.webmd.com/drugs/2/drug-522-2202/magnesium-citrate-oral/magnesium-citrate-oral/details.
- ↑ "Magnesium". Office of Dietary Supplements, National Institutes of Health. https://ods.od.nih.gov/factsheets/magnesium-HealthProfessional/.
- ↑ "Magnesium Citrate". https://medlineplus.gov/druginfo/meds/a619019.html.
- ↑ Schuchardt, Jan Philipp; Hahn, Andreas (22 September 2017). "Intestinal Absorption and Factors Influencing Bioavailability of Magnesium- An Update". Current Nutrition & Food Science 13 (4): 260–278. doi:10.2174/1573401313666170427162740. PMID 29123461.
- ↑ Coudray, Charles C.; Rambeau, Mathieu; Feillet Coudray, Christine; Gueux, Elyette; Tressol, Jean-Claude; Mazur, André; Rayssiguier, Yves (2005). "Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach". Magnesium Research 18 (4): 215–223. PMID 16548135. https://hal.science/hal-02682984/.
- ↑ Ettinger, B; Pak, CY; Citron, JT; Thomas, C; Adams-Huet, B; Vangessel, A (December 1997). "Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis". J Urol 158 (6): 2069–73. doi:10.1016/S0022-5347(01)68155-2. PMID 9366314.
- ↑ Dhaliwal, Sean (August 2025). "A Guide to Common Magnesium Citrate Side Effects". https://www.ingredientsonline.com/blogs/magnesium-citrate-side-effects-guide/.
- ↑ magnesium citrate. Cerner Multum. Drugs.com. 12 April 2009.
External links
- Saline laxatives. MedicineNet.
- Magnesium citrate Patient Advice. Drugs.com.
 |
