Chemistry:Motesanib
| |
| Names | |
|---|---|
| Preferred IUPAC name
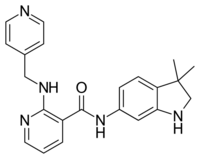
N-(3,3-Dimethyl-2,3-dihydro-1H-indol-6-yl)-2-{[(pyridin-4-yl)methyl]amino}pyridine-3-carboxamide | |
| Other names
AMG 706
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H23N5O | |
| Molar mass | 373.460 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Motesanib (AMG 706) is an experimental drug candidate originally developed by Amgen[1] but later investigated by the Takeda Pharmaceutical Company. It is an orally administered small molecule belonging to angiokinase inhibitor class which acts as an antagonist of VEGF receptors, platelet-derived growth factor receptors, and stem cell factor receptors.[2] It is used as the phosphate salt motesanib diphosphate. After clinical trials in thyroid cancer, non-small cell lung cancer, gastrointestinal stromal cancer, colorectal cancer, and breast cancer, the drug was not found to show sufficient efficacy for further development, and development was abandoned by Takeda.[3]
Clinical trials
Motesanib was originally investigated for effectiveness against advanced nonsquamous non-small-cell lung cancer (NSCLC), with Phase II trials indicating an effectiveness comparable to bevacizumab when they were both used in combination with paclitaxel/carboplatin.[4] However a later and more detailed Phase III trial failed to show any benefit for the treatment of NSCLC.[2][5] A second Phase III trial was started in 2012,[6] which focused on patients from Asian backgrounds (performed on the basis of subgroup analysis)[7] however this also failed to meet its primary endpoint.[8]
The drug has undergone a Phase II evaluation as first-line therapy for breast cancer[2] however this study found no evidence to support further investigation.[9] Phase II testing against persistent or recurrent ovarian, fallopian tube and primary peritoneal carcinomas was also unsuccessful.[10] Two phase II clinical trials for thyroid cancer showed promising results.[11][12][13]
References
- ↑ Stafford, edited by Rongshi Li, Jeffrey A. (2009). "Chapter 5. Discovery of Motesanib". Kinase inhibitor drugs. Hoboken, N.J.: Wiley. pp. 113–130. doi:10.1002/9780470524961.ch5. ISBN 978-0-470-27829-1. https://archive.org/details/kinaseinhibitord00liro.
- ↑ 2.0 2.1 2.2 "Amgen and Takeda's NSCLC Drug Fails in Phase III Study". 30 Mar 2011. http://www.genengnews.com/gen-news-highlights/amgen-and-takeda-s-nsclc-drug-fails-in-phase-iii-study/81244902/.
- ↑ "Motesanib". http://adisinsight.springer.com/drugs/800011016.
- ↑ Blumenschein Jr, G. R.; Kabbinavar, F.; Menon, H.; Mok, T. S. K.; Stephenson, J.; Beck, J. T.; Lakshmaiah, K.; Reckamp, K. et al. (14 February 2011). "A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer". Annals of Oncology 22 (9): 2057–2067. doi:10.1093/annonc/mdq731. PMID 21321086.
- ↑ Scagliotti, G. V.; Vynnychenko, I.; Park, K.; Ichinose, Y.; Kubota, K.; Blackhall, F.; Pirker, R.; Galiulin, R. et al. (2 July 2012). "International, Randomized, Placebo-Controlled, Double-Blind Phase III Study of Motesanib Plus Carboplatin/Paclitaxel in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer: MONET1". Journal of Clinical Oncology 30 (23): 2829–2836. doi:10.1200/JCO.2011.41.4987. PMID 22753922. https://iris.unito.it/bitstream/2318/118283/1/Scagliotti-motesanib.pdf.
- ↑ "Takeda Initiates Phase 3 Trial of Motesanib in Japan and Additional Asian Countries". Takeda Pharmaceutical Company Limited. http://www.takeda.com/news/2012/20120726_3985.html.
- ↑ Kubota, K.; Ichinose, Y.; Scagliotti, G.; Spigel, D.; Kim, J. H.; Shinkai, T.; Takeda, K.; Kim, S.- W. et al. (13 January 2014). "Phase III study (MONET1) of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous nonsmall-cell lung cancer (NSCLC): Asian subgroup analysis". Annals of Oncology 25 (2): 529–536. doi:10.1093/annonc/mdt552. PMID 24419239.
- ↑ "Takeda Announces Phase 3 MONET-A Study Evaluating Motesanib (AMG 706) in Patients with Advanced Non-Squamous Non-Small Cell Lung Cancer Does Not Meet Primary Endpoint". Takeda Pharmaceutical Company Limited. http://www.takeda.com/news/2015/20150217_6909.html.
- ↑ Martin, Miguel; Roche, Henri; Pinter, Tamas; Crown, John; Kennedy, M John; Provencher, Louise; Priou, Frank; Eiermann, Wolfgang et al. (April 2011). "Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study". The Lancet Oncology 12 (4): 369–376. doi:10.1016/S1470-2045(11)70037-7. PMID 21429799.
- ↑ Schilder, R.J.; Sill, M.W.; Lankes, H.A.; Gold, M.A.; Mannel, R.S.; Modesitt, S.C.; Hanjani, P.; Bonebrake, A.J. et al. (April 2013). "A phase II evaluation of motesanib (AMG 706) in the treatment of persistent or recurrent ovarian, fallopian tube and primary peritoneal carcinomas: A Gynecologic Oncology Group study". Gynecologic Oncology 129 (1): 86–91. doi:10.1016/j.ygyno.2013.01.006. PMID 23321064.
- ↑ Motesanib Diphosphate Provides Anticancer Activity Among Patients with Progressive Thyroid Cancer, CancerConnect.com
- ↑ Schlumberger, M. J.; Elisei, R.; Bastholt, L.; Wirth, L. J.; Martins, R. G.; Locati, L. D.; Jarzab, B.; Pacini, F. et al. (29 June 2009). "Phase II Study of Safety and Efficacy of Motesanib in Patients With Progressive or Symptomatic, Advanced or Metastatic Medullary Thyroid Cancer". Journal of Clinical Oncology 27 (23): 3794–3801. doi:10.1200/JCO.2008.18.7815. PMID 19564535.
- ↑ Sherman, Steven I.; Wirth, Lori J.; Droz, Jean-Pierre; Hofmann, Michael; Bastholt, Lars; Martins, Renato G.; Licitra, Lisa; Eschenberg, Michael J. et al. (3 July 2008). "Motesanib Diphosphate in Progressive Differentiated Thyroid Cancer". New England Journal of Medicine 359 (1): 31–42. doi:10.1056/NEJMoa075853. PMID 18596272.
External links
 |
