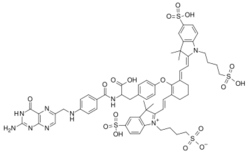
Chemistry:Pafolacianine
 | |
| Clinical data | |
|---|---|
| Trade names | Cytalux |
| Other names | OTL38, OTL-0038 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C61H67N9O17S4 |
| Molar mass | 1326.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pafolacianine, sold under the brand name Cytalux, is an optical imaging agent used in fluorescence-guided surgery.[1][2][3][4][5][6] Pafolacianine is a fluorescent medication that binds to folate receptor (FR)-expressing cells.[1]
The most common side effects of pafolacianine include infusion-related reactions, including nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, itching and hypersensitivity.[2][4]
It was approved for medical use in the United States in November 2021.[2][7][6] The U.S. Food and Drug Administration considers it to be a first-in-class medication.[8]
Medical uses
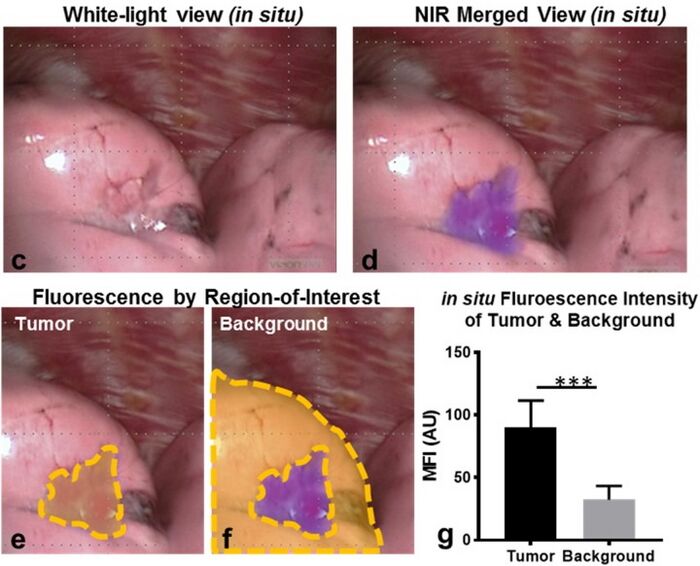
Pafolacianine is indicated as an adjunct for intraoperative identification of malignant lesions in people with ovarian cancer.[1][2] It is also indicated to assist identifying lung cancer lesions in adults with known or suspected lung cancer.[3]
History
Scientists from Purdue University designed and developed OTL38 and licensed it to On Target Laboratories in 2013.[9][10] The safety and effectiveness of pafolacianine was evaluated in a randomized, multi-center, open-label study of women diagnosed with ovarian cancer or with high clinical suspicion of ovarian cancer who were scheduled to undergo surgery.[2][4] Of the 134 women (ages 33 to 81 years) who received a dose of pafolacianine and were evaluated under both normal and fluorescent light during surgery, 26.9% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.[2][4]
The safety and effectiveness of pafolacianine was evaluated in a randomized, multicenter, open-label study (NCT04241315) of participants with known or suspected lung cancer who were scheduled to undergo surgery.[3] Of the 110 participants who received a dose of pafolacianine and were evaluated under both normal and fluorescent light during surgery, 24% had at least one cancerous lesion detected that was not observed by standard visual or tactile inspection.[3]
The U.S. Food and Drug Administration (FDA) granted the application for pafolacianine orphan drug, priority review, and fast track designations.[2][4][11][8] The FDA granted the approval of Cytalux to On Target Laboratories, LLC.[2][3]
Figures
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 "Cytalux- pafolacianine injection injection". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d21952b9-7c4e-3a56-e053-2a95a90a0ab0.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "FDA Approves New Imaging Drug to Help Identify Ovarian Cancer Lesions". U.S. Food and Drug Administration (FDA) (Press release). 29 November 2021. Retrieved 30 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 "FDA approves imaging drug to help identify lung cancer lesions". U.S. Food and Drug Administration (FDA) (Press release). 16 December 2022. Retrieved 16 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 "FDA approves pafolacianine for identifying malignant ovarian cancer lesions". 1 December 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pafolacianine-identifying-malignant-ovarian-cancer-lesions.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Identification of a Folate Receptor-Targeted Near-Infrared Molecular Contrast Agent to Localize Pulmonary Adenocarcinomas". Molecular Therapy 26 (2): 390–403. February 2018. doi:10.1016/j.ymthe.2017.10.016. PMID 29241970.
- ↑ Jump up to: 6.0 6.1 "The complementary value of intraoperative fluorescence imaging and Raman spectroscopy for cancer surgery: combining the incompatibles". European Journal of Nuclear Medicine and Molecular Imaging 49 (7): 2364–2376. June 2022. doi:10.1007/s00259-022-05705-z. PMID 35102436.
- ↑ "On Target Laboratories Announces FDA Approval of Cytalux (pafolacianine) injection for Identification of Ovarian Cancer During Surgery". On Target Laboratories. 29 November 2021. https://www.prnewswire.com/news-releases/on-target-laboratories-announces-fda-approval-of-cytalux-pafolacianine-injection-for-identification-of-ovarian-cancer-during-surgery-301433036.html.
- ↑ Jump up to: 8.0 8.1 (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Synthesis and composition of amino acid linking groups conjugated to compounds used for the targeted imaging of tumors". https://patents.google.com/patent/US9061057B2/en?oq=9061057.
- ↑ "Synthesis and composition of amino acid linking groups conjugated to compounds used for the targeted imaging of tumors". https://patents.google.com/patent/US9333270B2/en?oq=9333270.
- ↑ "Pafolacianine Orphan Drug Designations and Approvals". 23 December 2014. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=458714.
- ↑ "An open label trial of folate receptor-targeted intraoperative molecular imaging to localize pulmonary squamous cell carcinomas". Oncotarget 9 (17): 13517–13529. March 2018. doi:10.18632/oncotarget.24399. PMID 29568374.
External links
- "Pafolacianine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/pafolacianine.
- Clinical trial number NCT03180307 for "OTL38 for Intra-operative Imaging of Folate Receptor Positive Ovarian Cancer" at ClinicalTrials.gov
- Clinical trial number NCT04241315 for "ELUCIDATE: Enabling Lung Cancer Identification Using Folate Receptor Targeting (Elucidate)" at ClinicalTrials.gov
 |

