Chemistry:Perrhenic acid
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetraoxorhenic(VII) acid
| |
| Other names
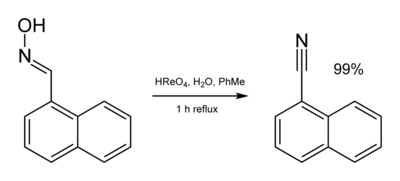
Hydrated rhenium(VII) oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| |
| |
| Properties | |
| H 4Re 2O 9 (solid) HReO 4 (gas) | |
| Molar mass | 251.2055 g/mol |
| Appearance | Pale yellow solid |
| Boiling point | sublimes |
| Soluble | |
| Acidity (pKa) | -1.25[1] |
| Conjugate base | Perrhenate |
| Structure | |
| octahedral-tetrahedral (solid) tetrahedral (gas) | |
| Hazards | |
| Main hazards | Corrosive |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P330, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perrhenic acid is the chemical compound with the formula Re
2O
7(H
2O)
2. It is obtained by evaporating aqueous solutions of Re
2O
7. Conventionally, perrhenic acid is considered to have the formula HReO
4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam.[2] When a solution of Re
2O
7 is kept for a period of months, it breaks down and crystals of HReO
4 · H2O are formed, which contain tetrahedral ReO−
4.[3] For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
Properties
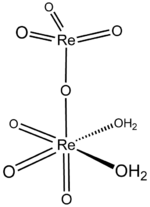
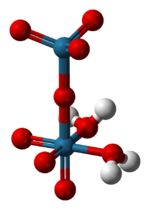
The structure of solid perrhenic acid is [O
3Re–O–ReO
3(H
2O)
2].[4] This species is a rare example of a metal oxide coordinated to water; most often metal–oxo–aquo species are unstable with respect to their corresponding hydroxides:
- M(O)(H
2O) → M(OH)
2
The two rhenium atoms have different bonding geometries, with one being tetrahedral and the other octahedral, and with the water ligands coordinated to the latter.
Gaseous perrhenic acid is tetrahedral, as suggested by its formula HReO
4.
Reactions
Perrhenic acid or the related anhydrous oxide Re
2O
7 converts to dirhenium heptasulfide upon treatment with hydrogen sulfide:
- Re
2O
7 + 7 H
2S → Re
2S
7 + 7 H
2O
The heptasulfide catalyzes various reductions.[5]
Perrhenic acid in the presence of hydrochloric acid undergoes reduction in the presence of thioethers and tertiary phosphines to give rhenium(V) complexes with the formula ReOCl
3L
2.[6]
Perrhenic acid combined with platinum on a support gives rise to a useful hydrogenation and hydrocracking catalyst for the petroleum industry.[7] For example, silica impregnated with a solution of perrhenic acid is reduced with hydrogen at 500 °C.[citation needed] This catalyst is used in the dehydrogenation of alcohols and also promotes the decomposition of ammonia.
Catalysis
Perrhenic acid is a precursor to a variety of homogeneous catalysts, some of which are promising in niche applications that can justify the high cost of rhenium. In combination with tertiary arsines, perrhenic acid gives a catalyst for the epoxidation of alkenes with hydrogen peroxide.[8] Perrhenic acid catalyses the dehydration of oximes to nitriles.[9]
Other uses
Perrhenic acid is also used in the manufacture of x-ray targets.[10][11]
See also
References
- ↑ http://www.iupac.org/publications/pac/1998/pdf/7002x0355.pdf [bare URL PDF]
- ↑ Glemser, O.; Müller, A.; Schwarzkopf, H. (1964). "Gasförmige Hydroxide. IX. Über ein Gasförmiges Hydroxid des Rheniums" (in German). Zeitschrift für anorganische und allgemeine Chemie 334 (1–2): 21–26. doi:10.1002/zaac.19643340105..
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Beyer, H.; Glemser, O.; Krebs, B. "Dirhenium Dihydratoheptoxide Re2O7(OH2)2 – New Type of Water Bonding in an Aquoxide" Angewandte Chemie, International Edition English 1968, Volume 7, Pages 295 - 296. doi:10.1002/anie.196802951.
- ↑ Schwarz, D. E.; Frenkel, A. I.; Nuzzo, R. G.; Rauchfuss, T. B.; Vairavamurthy, A. (2004). "Electrosynthesis of ReS4. XAS Analysis of ReS2, Re2S7, and ReS4". Chemistry of Materials 16: 151–158. doi:10.1021/cm034467v.
- ↑ Parshall, G. W.; Shive, L. W.; Cotton, F. A. (1997). Phosphine Complexes of Rhenium. Inorganic Syntheses. 17. pp. 110–112. doi:10.1002/9780470132487.ch31. ISBN 9780470132487.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ van Vliet, M. C. A.; Arends, I. W. C. E.; Sheldon, R. A. (1999). "Rhenium Catalysed Epoxidations with Hydrogen Peroxide: Tertiary Arsines as Effective Cocatalysts". J. Chem. Soc., Perkin Trans. 1 (3): 377–80. doi:10.1039/a907975k.
- ↑ Ishihara, K.; Furuya, Y.; Yamamoto, H. (2002). "Rhenium(VII) Oxo Complexes as Extremely Active Catalysts in the Dehydration of Primary Amides and Aldoximes to Nitriles". Angewandte Chemie International Edition 41 (16): 2983–2986. doi:10.1002/1521-3773(20020816)41:16<2983::AID-ANIE2983>3.0.CO;2-X. PMID 12203432.
- ↑ http://www.gehealthcare.com/usen/service/time_material_support/docs/Radplus2100.pdf[yes|permanent dead link|dead link}}]
- ↑ X-ray
 |