Chemistry:Sodium perrhenate
| |

| |
| Names | |
|---|---|
| Other names
Sodium rhenate(VII)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
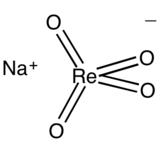
| NaReO4 | |
| Molar mass | 273.1866 g/mol |
| Appearance | white solid |
| Density | 5.39 g/cm3 |
| Melting point | 414 °C (777 °F; 687 K) |
| 103.3 g/100 mL (0 °C) 114.0 g/100 mL (25 °C)[1] 145.3 g/100 mL (30 °C) 173.0 g/100 mL (50 °C) | |
| Solubility | soluble in water (> 1130 g/L at 25 °C)[1] |
| Structure | |
| tetragonal | |
| Hazards | |
| Main hazards | Oxidizer, skin/eyes irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.
Preparation
It can be prepared by treatment of rhenium heptoxide with base or by ion exchange from the potassium salt.[2]
Sodium perrhenate can be prepared from rhenium metal with hydrogen peroxide in the presence of base.[3]
- [math]\ce{ 2 Re + 7 H2O2 + 2 NaOH -> 2 NaReO4 + 8 H2O }[/math]
Reactions
It reacts with sodium in ethanol to give nonahydridorhenate.[2]
Sodium perrhenate has been used as a precursor of rhenium nitrides (such as Re3N, Re2N, Re3N2, ReN2, ReN3, ReN4), which can be used as catalysts for ammonia synthesis and for hydro-denitrogenation.[4]
It can be used to prepare Re2(CO)10.[3]
References
- ↑ 1.0 1.1 Luis Cifuentes, J. M. Casas (February 2012). "Crystallization of Sodium Perrhenate from NaReO4–H2O–C2H5OH Solutions at 298 K". Hydrometalurgy 113-114: 192–194. doi:10.1016/j.hydromet.2011.12.022. Bibcode: 2012HydMe.113..192C.
- ↑ 2.0 2.1 A. P. Ginsberg; C. R. Sprinkle (1972). "Nonahydridorhenate Salts". Inorganic Syntheses. 13. pp. 219–225. doi:10.1002/9780470132449.ch45. ISBN 978-0-470-13244-9.
- ↑ 3.0 3.1 Crocker, Lisa S.; Gould, George L.; Heinekey, D. Michael (1988). "Improved Synthesis of Carbonylrhenium". Journal of Organometallic Chemistry 342 (2): 243–244. doi:10.1016/s0022-328x(00)99461-0.
- ↑ Hämäläinen, Jani; Mizohata, Kenichiro; Meinander, Kristoffer; Mattinen, Miika; Vehkamäki, Marko; Räisänen, Jyrki; Ritala, Mikko; Leskelä, Markku (2018-08-27). "Rhenium Metal and Rhenium Nitride Thin Films Grown by Atomic Layer Deposition" (in en). Angewandte Chemie International Edition 57 (44): 14538–14542. doi:10.1002/anie.201806985. ISSN 1433-7851. PMID 30048031.
Further reading
- Ahluwalia, J. C.; Cobble, J. W. (1 December 1964). "The Thermodynamic Properties of High Temperature Aqueous Solutions. II. Standard Partial Molal Heat Capacities of Sodium Perrhenate and Perrhenic Acid from 0 to 100o". Journal of the American Chemical Society 86 (24): 5377–5381. doi:10.1021/ja01078a001.
- Dwek, Raymond A.; Luz, Z.; Shporer, M. (1 May 1970). "Nuclear magnetic resonance of aqueous solutions of sodium perrhenate". The Journal of Physical Chemistry 74 (10): 2232–2233. doi:10.1021/j100909a038.
 |

