Chemistry:Phenacene
Phenacenes are a class of organic compounds consisting of fused aromatic rings. They are polycyclic aromatic hydrocarbons, related to acenes and helicenes from which they differ by the arrangement of the fused rings.
| [n]Phenacene | Common name | Structure |
|---|---|---|
| [4]phenacene | Chrysene | 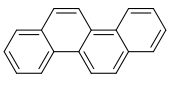
|
| [5]phenacene | Picene | 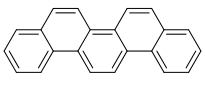
|
| [6]phenacene | Fulminene | Error creating thumbnail: Unable to save thumbnail to destination |
| [7]phenacene | 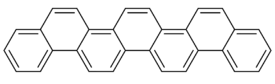
|
Relevance to organic electronic materials
Aromatic compounds with extended π-conjugated system have attracted attention because of their potential utilization in organic electronics as organic semiconductors.[1] Of academic interest, pentacene has been widely used as an active layer in organic thin film field effect transistors (OFET). The main drawback of pentacene OFET is degradation upon exposure to light and air. On the other hand, [n]phenacenes, an isomeric form of [n]acenes, has been known as a stable compound in which the benzene rings are fused in a zigzag structure. For the past several years, there is renewed interest in synthesis of [n]phenacene derivatives associated with electronic applications in emissive and semi- or superconducting materials.[2][3][4]
Picene ([5]phenacene) can serve as an active layer of a high performance p-channel organic thin film FET with very high field-effect mobility: μ of 5 cm2∙V−1∙s−1.[5] [7]Phenacene FET shows effect mobility μ of 0.75 cm2∙V−1∙s−1 and no sensitivity to air. Furthermore, picene doped with potassium and rubidium exhibit superconductivity with a maximum critical temperature 𝑇𝑐∼18 K.[4] Thus, [n]phenacenes and their derivatives may play an important role in future fabrication of stable and high-performance electronic devices such as OFET, OLED and organic solar cells. Substituted picenes may serve as an active layer of OFETs.[6]
References
- ↑ Yamashita, Yoshiro (2009). "Organic semiconductors for organic field-effect transistors". Science and Technology of Advanced Materials 10 (2): 024313. doi:10.1088/1468-6996/10/2/024313. ISSN 1468-6996. PMID 27877286. Bibcode: 2009STAdM..10b4313Y.
- ↑ Komura, N.; Goto, H.; He, X.; Mitamura, H.; Eguchi, R.; Kaji, Y.; Okamoto, H.; Sugawara, Y. et al. (2012). "Characteristics of [6]phenacene thin film field-effect transistor". Appl. Phys. Lett. 101 (8): 083301. doi:10.1063/1.4747201. Bibcode: 2012ApPhL.101h3301K.
- ↑ Ionkin, A. S.; Marshall, W. J.; Fish, B. M.; Bryman, L. M.; Wang, Y. (2008). "A tetra-substituted chrysene: orientation of multiple electrophilic substitution and use of a tetra-substituted chrysene as a blue emitter for OLEDs". Chem. Commun. (20): 2319. doi:10.1039/b715386d.
- ↑ 4.0 4.1 Mitsuhashi, R.; Suzuki, Y.; Yamanari, Y.; Mitamura, H.; Kambe, T.; Ikeda, N.; Okamoto, H.; Fujiwara, A. et al. (2010). "Superconductivity in alkali-metal-doped picene". Nature 464 (7285): 76–9. doi:10.1038/nature08859. PMID 20203605. Bibcode: 2010Natur.464...76M.
- ↑ Okamoto, H.; Kawasaki, N.; Kaji, Y.; Kubozono, Y.; Fujiwara, A.; Yamaji, M. (2008). "Air-assisted high-performance field-effect transistor with thin films of picene". J. Am. Chem. Soc. 130 (32): 10470–1. doi:10.1021/ja803291a. PMID 18627146.
- ↑ Nakano, Y.; Saito, M.; Nakamura, H. WO 2010016511 A1 20100211, 2010.
 |
