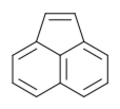
Chemistry:Acenaphthylene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acenaphthylene[1] | |||
| Other names
Cyclopenta[de]naphthalene
Acenaphthalene Tricyclo[6.3.1.04,12]dodeca-1(12),2,4,6,8,10-hexaene[citation needed] Tricyclo[6.3.1.04,12]dodecahexaene[citation needed] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C12H8 | |||
| Molar mass | 152.196 g·mol−1 | ||
| Appearance | Yellow crystals | ||
| Density | 0.8987 g cm−3 | ||
| Melting point | 91.8 °C (197.2 °F; 364.9 K) | ||
| Boiling point | 280 °C (536 °F; 553 K) | ||
| Insoluble | |||
| Solubility in ethanol | very soluble | ||
| Solubility in diethyl ether | very soluble | ||
| Solubility in benzene | very soluble | ||
| Solubility in chloroform | soluble | ||
| Thermochemistry[1][2] | |||
Heat capacity (C)
|
166.4 J mol−1 K−1 | ||
Enthalpy of fusion (ΔfH⦵fus)
|
186.7 kJ/mol | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H302, H310, H315, H319, H330, H335 | |||
| P260, P261, P262, P264, P270, P271, P280, P284, P301+312, P302+350, P302+352, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P332+313, P337+313, P361, P362, P363, P403+233 | |||
| Flash point | 122 °C (252 °F; 395 K) | ||
| Related compounds | |||
Related compounds
|
acenaphthene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid.[3] Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.
Occurrence
Acenaphthylene occurs as about 2% of coal tar. It is produced industrially by gas phase dehydrogenation of acenaphthene.[3]
Reactions
Hydrogenation gives the more saturated compound acenaphthene. Chemical reduction affords the radical anion sodium or potassium acenaphthalenide, which is used as a strong reductant (E = -2.26 V vs FC).[4]
It functions as a ligand for some organometallic compounds.[5]
Uses
Polymerisation of acenaphthylene with acetylene in the presence of a Lewis acid catalyst gives electrically conductive polymers. Acenaphthylene possesses excellent properties as an antioxidant in cross-linked polyethylene and ethylene-propylene rubber. Thermal trimerization of acenaphthylene leads to decacyclene, which can be further processed to sulfur dyes.[6]
Toxicity
The no-observed-effect-level of acenaphthylene after repeated 28-day oral administration to both male and female rats was found to be 4 mg/kg/day.[7]
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 210. doi:10.1039/9781849733069-00130. ISBN 978-0-85404-182-4.
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–3. ISBN 978-1138561632.
- ↑ 3.0 3.1 Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
- ↑ Connelly, Neil G.; Geiger, William E. (1996-01-01). "Chemical Redox Agents for Organometallic Chemistry" (in en). Chemical Reviews 96 (2): 877–910. doi:10.1021/cr940053x. ISSN 0009-2665. PMID 11848774. https://pubs.acs.org/doi/10.1021/cr940053x.
- ↑ Motoyama, Yukihiro; Itonaga, Chikara; Ishida, Toshiki; Takasaki, Mikihiro; Nagashima, Hideo (2005). "Catalytic Reduction of Amides to Amines with Hydrosilanes Using a Triruthenium Carbonyl Cluster as the Catalyst". Organic Syntheses 82: 188. doi:10.15227/orgsyn.082.0188. http://orgsyn.org/demo.aspx?prep=v82p0188.
- ↑ Ullmann, 4th ed., 21, 70
- ↑ Tanabe, S. (2017). "Toxicity of repeated 28-day oral administration of acenaphthylene in rats". Fundamental Toxicological Sciences 4 (6): 247–259. doi:10.2131/fts.4.247.
 |