Chemistry:Potassium ethyl xanthate
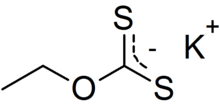
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Potassium O-ethylcarbonodithioate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
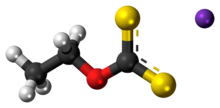
| CH 3CH 2OCS 2K | |
| Molar mass | 160.29 g·mol−1 |
| Appearance | Pale yellow powder |
| Density | 1.263 g/cm3[1] |
| Melting point | 225 to 226 °C (437 to 439 °F; 498 to 499 K) |
| Boiling point | decomposes |
| Acidity (pKa) | approximately 1.6 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H302, H315, H319, H332, H335 | |
| P210, P240, P241, P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P370+378, P403+233, P405, P501 | |
| Related compounds | |
Other cations
|
Sodium ethyl xanthate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH
3CH
2OCS
2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. It is a potassium salt of ethyl xanthic acid.
Production and properties
Xanthate salts are prepared by the action of alkoxides on carbon disulfide. The alkoxide is often generated in situ from potassium hydroxide:[2]
- CH
3CH
2OH + CS
2 + KOH → CH
3CH
2OCS
2K + H
2O
Potassium ethyl xanthate is a pale yellow powder that is stable at high pH, but rapidly hydrolyses below pH = 9:
- CH
3CH
2OCS
2K + H+
→ CH
3CH
2OH + CS
2 + K+
Oxidation gives diethyl dixanthogen disulfide:
- 4 CH
3CH
2OCS
2K + 2 H
2O + O
2 → 2 (CH
3CH
2OCS
2)
2 + 4 KOH
KEX is a source of ethylxanthate coordination complexes. For example (CH
3CH
2OCS
2)
3M have been prepared from KEX for M = Cr, In, Co.[clarification needed][3]
Applications
Potassium ethyl xanthate is used in the mining industry as flotation agent for extraction of the ores of copper, nickel, and silver.[4] The method exploits the affinity of these "soft" metals for the organosulfur ligand.
Potassium xanthate is a useful reagent for preparing xanthate esters from alkyl and aryl halides. The resulting xanthate esters are useful intermediates in organic synthesis.[5]
Safety
The LD50 is 103 mg/kg (oral, rats) for potassium ethyl xanthate.[4]
References
- ↑ Report 5 (1995) p. 5
- ↑ This report gives a detailed recipe for potassium ethyl xanthate: Charles C. Price, Gardner W. Stacy (1948). "p-Nitrophenyl) Sulfide". Organic Syntheses 28: 82. doi:10.15227/orgsyn.028.0082.
- ↑ Galsbøl, F.; Schäffer, C. E. (1967). "Tris (O-Ethyldithiocarbonato) Complexes of Tripositive Chromium, Indium, and Cobalt". Inorganic Syntheses 10: 42–49. doi:10.1002/9780470132418.ch6.
- ↑ 4.0 4.1 Kathrin-Maria Roy (2005). "Xanthates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_423. ISBN 3527306730.
- ↑ One of several procedures using xanthate esters: Fabien Gagosz and Samir Z. Zard (1948). "A Xanthate-Transfer Approach to α-Trifluoromethylamines". Organic Syntheses 84: 32. http://www.orgsyn.org/demo.aspx?prep=V84P0032.; Collective Volume, 11, pp. 212
 |

