Chemistry:Praseodymium antimonide
From HandWiki
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| PrSb | |
| Molar mass | 262.67 g/mol |
| Density | 6.7 g/cm3 |
| Melting point | 2161 or 2170 °C |
| Related compounds | |
Other anions
|
PrN, PrP, PrAs, PrBi, Pr2O3 |
Other cations
|
CeSb, NdSb |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Praseodymium antimonide is a binary inorganic compound of praseodymium and antimony with the formula PrSb.
Preparation
Praseodymium antimonide can be prepared by heating praseodymium and antimony in a vacuum:
- [math]\displaystyle{ \mathsf{ Pr + Sb \ \xrightarrow{2170^oC}\ PrSb } }[/math]
Physical properties
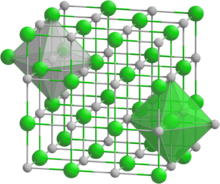
Praseodymium antimonide forms cubic crystals, space group Fm3m, cell parameters a = 0.638 nm, Z = 4, and structure like sodium chloride.[1][2][3]
The compound melts congruently at 2170 °C[1] or 2161 °C.[2] At a temperature of 1950 °C, a phase transition occurs in the crystals. At a pressure of 13 GPa, a phase transition also occurs.[4]
See also
References
- ↑ 1.0 1.1 Diagrammy sostojanija dvojnych metalličeskich sistem: spravočnik v trech tomach. 3,2. Moskva: Mašinostroenie. 2001. ISBN 978-5-217-02932-7.
- ↑ 2.0 2.1 Franke, P.; Neuschütz, D.; Scientific Group Thermodata Europe (SGTE) (2006), Franke, P.; Neuschütz, D., eds., "Pr-Sb" (in en), Binary Systems. Part 4: Binary Systems from Mn-Mo to Y-Zr (Berlin/Heidelberg: Springer-Verlag) 19B4: pp. 1–4, doi:10.1007/10757285_56, ISBN 978-3-540-25024-1, http://materials.springer.com/lb/docs/sm_lbs_978-3-540-32593-2_56, retrieved 2023-06-20
- ↑ Predel, B. (1998), Madelung, O., ed., "Pr-Sb (Praseodymium-Antimony)" (in en), Ni-Np – Pt-Zr, Landolt-Börnstein - Group IV Physical Chemistry (Berlin/Heidelberg: Springer-Verlag) I: pp. 1–2, doi:10.1007/10542753_2498, ISBN 978-3-540-61712-9, http://materials.springer.com/lb/docs/sm_lbs_978-3-540-70692-2_2498, retrieved 2023-06-20
- ↑ Gupta, Dinesh Chandra; Raypuria, Gajendra Singh (January 2013). "Phase Transition of Praseodymium Mono-Pnictides Under High Pressure" (in en). International Journal of Modern Physics: Conference Series 22: 491–496. doi:10.1142/S2010194513010568. ISSN 2010-1945. Bibcode: 2013IJMPS..22..491G.
 |

