Chemistry:Antimony sulfate
| |
| Names | |
|---|---|
| IUPAC name
Antimony(3+) trisulfate
| |
| Other names
Antimony(III) sulfate
Antimonous sulfate Antimony trisulfate Diantimony trisulfate Diantimony tris(sulphate) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[2] | |
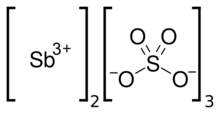
| Sb2(SO4)3 | |
| Molar mass | 531.7078 g/mol |
| Density | 3.6246 g/cm3[1] |
| soluble | |
| Hazards | |
| Safety data sheet | MSDS |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[3] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.[1]
Structure
Solid antimony sulfate contains infinite ladders of SO4 tetrahedra and SbO3 pyramids sharing corners. It is often described as a mixed oxide, Sb2O3.3SO3.[4]
Chemical properties
Antimony sulfate is sometimes called a "salt" as it can be produced from the reaction of antimony and sulfuric acid, but antimony does not form a nitrate when dissolved in nitric acid, (an oxidising acid) but produces a mixture of antimony oxides, and this contrasts with bismuth which dissolves in both acids to form salts.[5] It is deliquescent, and soluble in acids. It can be prepared by dissolving antimony, antimony trioxide, antimony trisulfide or antimony oxychloride in hot, concentrated sulfuric acid.[1][5]
- 2 Sb (s) + 6 H2SO4 → Sb2(SO4)3 + 3SO2 + 6 H2O
Uses
Owing to its solubility, antimony sulfate has uses in the doping of semiconductors.[6] It is also used for coating anodes in electrolysis and in the production of explosives and fireworks.[1]
Safety
Antimony(III) sulfate causes irritation to the skin and mucous membranes.[7]
Natural occurrence
Natural analogue of the exact compound is yet unknown. However, basic hydrated Sb sulfates are known as the minerals klebelsbergite[8][9] and coquandite.[10][11]
References
- ↑ 1.0 1.1 1.2 1.3 Herbst, Karl Albert et al. (1985) Antimony and antimony compounds in Ullmann's Encyclopedia of Industrial Chemistry 5th ed., vol. A3, p. 70. ISBN 3-527-20103-3.
- ↑ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- ↑ 3.0 3.1 NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0036.html.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ 5.0 5.1 Nicholas C. Norman (31 December 1997). Chemistry of arsenic, antimony, and bismuth. Springer. pp. 193–. ISBN 978-0-7514-0389-3. https://books.google.com/books?id=vVhpurkfeN4C&pg=PA193.
- ↑ Method of forming phase change layer, method of manufacturing a storage node using the same, and method of manufacturing phase change memory device using the same – Samsung Electronics Co., Ltd. Freepatentsonline.com (2007-01-02). Retrieved on 2011-12-23.
- ↑ Antimony(III) Sulfate Material Safety Data Sheet . Prochemonline.
- ↑ https://www.mindat.org/min-2223.html
- ↑ https://www.ima-mineralogy.org/Minlist.htm
- ↑ https://www.mindat.org/min-1125.html
- ↑ https://www.ima-mineralogy.org/Minlist.htm
