Chemistry:Romanèchite
| Romanèchite | |
|---|---|
 Romanèchite, from the Marquette County, Michigan, US. | |
| General | |
| Category | Oxide minerals |
| Formula (repeating unit) | (Ba,H 2O) 2(Mn4+,Mn3+) 5O 10 |
| Strunz classification | 4.DK.10 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | C2/m |
| Identification | |
| Mohs scale hardness | 6–6.5 |
| |re|er}} | Submetallic |
| Streak | Blue-black |
| References | [1][2][3] |
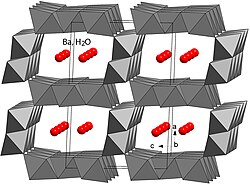
Romanèchite ((Ba,H
2O)
2(Mn4+,Mn3+)
5O
10) is the primary constituent of psilomelane, which is a mixture of minerals. Most psilomelane is not pure romanechite, so it is incorrect to consider them synonyms. Romanèchite is a valuable ore of manganese, which is used in steelmaking and sodium battery production.[6] It has a monoclinic crystal structure, a hardness of 6 and a specific gravity of 4.7–5. Romanèchite's structure consists of 2 × 3 tunnels formed by MnO6 octahedra.[5][7]
It is commonly associated with hematite, barite, pyrolusite, quartz and other manganese oxide minerals. It has been found in France , Germany , England , Brazil , Russia , India , and various parts of the United States , including Arizona, Virginia, Tennessee and Michigan, and sites throughout the Appalachian Valley and Ridge.[8] It occurs also in ferromanganese nodules from Lake Baikal.[9]
Bicolor (left) and tricolor (right) X-ray fluorescence maps of the distribution of Mn, Fe, and Ba in a ferromanganese nodule from Lake Baikal. Size = 5 mm (H) × 3 mm (V).[9]
References
- ↑ Romanechite, Webmineral.com
- ↑ Romanechite, Mindat.org
- ↑ "Oxides and Htdroxides.". Simon & Schuster's Guide to Rocks and Minerals.. New York: Simon & Schuster. 1977.
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine 85 (3): 291–320. doi:10.1180/mgm.2021.43. Bibcode: 2021MinM...85..291W.
- ↑ 5.0 5.1 Turner S and Post JE (1988) American Mineralogist 73: 1155–1161
- ↑ "Romanechite-structured Na(0.31)MnO(1.9) nanofibers as high-performance cathode material for a sodium-ion battery". Chemical Communications 51 (80): 14848–51. October 2015. doi:10.1039/C5CC05739F. PMID 26344149. https://pubs.rsc.org/en/content/articlelanding/2015/cc/c5cc05739f.
- ↑ "Manganese oxide minerals: crystal structures and economic and environmental significance". Proceedings of the National Academy of Sciences of the United States of America 96 (7): 3447–54. March 1999. doi:10.1073/pnas.96.7.3447. PMID 10097056. Bibcode: 1999PNAS...96.3447P.
- ↑ "New insight into the origin of manganese oxide ore deposits in the Appalachian Valley and Ridge of northeastern Tennessee and northern Virginia, USA" (in en). GSA Bulletin 129 (9–10): 1158–1180. 2017-09-01. doi:10.1130/B31682.1. ISSN 0016-7606. https://pubs.geoscienceworld.org/gsa/gsabulletin/article-abstract/129/9-10/1158/207612/New-insight-into-the-origin-of-manganese-oxide-ore.
- ↑ 9.0 9.1 Manceau A, Kersten M, Marcus MA, Geoffroy N. and Granina L (2007) "Ba and Ni speciation in a nodule of binary Mn oxide phase composition from Lake Baikal". Geochimica et Cosmochimica Acta, 71: 1967–1981. doi:10.1016/j.gca.2007.02.007
 |


