Chemistry:Rubidium sulfide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Rubidium sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Rb2S | |
| Molar mass | 203.00 |
| Appearance | white crystal |
| Density | 2.912 g/cm3[1] |
| Melting point | 530 °C[2] |
| hydrolyses to rubidium bisulfide[1] | |
| Solubility in ethanol and glycerol | soluble |
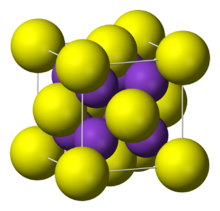
| Structure | |
| cubic:anti-fluorite | |
| Hazards | |
| Main hazards | toxic |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H314, H400 | |
| P260, P264, P273, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P391, P405, P501 | |
| Related compounds | |
Other anions
|
Rubidium oxide Rubidium selenide Rubidium telluride Rubidium polonide |
Other cations
|
Lithium sulfide Sodium sulfide Potassium sulfide Caesium sulfide Francium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rubidium sulfide is an inorganic compound and a salt with the chemical formula Rb2S. It is a white solid with similar properties to other alkali metal sulfides.
Production
By dissolving hydrogen sulfide into rubidium hydroxide solution, it will produce rubidium bisulfide, followed by rubidium sulfide.[3][4]
Properties
Physical properties
Rubidium sulfide has a cubic crystal similar to lithium sulfide, sodium sulfide and potassium sulfide, known as the anti-fluorite structure. Their space groups are . Rubidium sulfide has a crystal lattice unit cell dimension of = 765.0 pm.[1]
Chemical properties
Rubidium sulfide reacts with sulfur in hydrogen gas to form rubidium pentasulfide, Rb2S5.[4][5]
References
- ↑ 1.0 1.1 1.2 Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 ([1], p. 692, at Google Books).
- ↑ Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 ([2], p. 336, at Google Books).
- ↑ Wilhelm Blitz, Ernst Wilke-Dörfurt: "Über Sulfide des Rubidiums und Cäsiums" in Zeitschr. f. anorg. Chem. 1906. 48, S. 297–317. Volltext
- ↑ 4.0 4.1 R. Abegg, F. Auerbach: 'Handbuch der anorganischen Chemie'. Verlag S. Hirzel, Bd. 2, 1908. S. 430.Volltext
- ↑ Wilhelm Blitz, Ernst Wilke-Dörfurt: Ueber die Pentasulfide des Rubidiums und Cäsiums. In Ber. d. dt. chem. Ges. 1905, 38, 1, S. 123–130, doi:10.1002/cber.19050380114.
 |
