Chemistry:Tetrabromoauric acid
From HandWiki
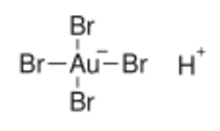
| |
| Names | |
|---|---|
| Other names
Tetrabromoauric(III) acid
Hydrogen tetrabromoaurate(III) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| H[AuBr 4] | |
| Molar mass | 517.591 g·mol−1 |
| Conjugate base | Tetrabromoaurate(III) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrabromoauric acid is an inorganic compound with the formula H[AuBr
4]. It is the bromide analog of chloroauric acid. It is generated analogously, by reacting a mixture of hydrobromic and nitric acids with elemental gold.[1][2] The oxidation state of gold in H[AuBr
4] and [AuBr
4]−
anion is +3. The salts of H[AuBr
4] (tetrabromoauric(III) acid) are tetrabromoaurates(III), containing [AuBr
4]−
anions (tetrabromoaurate(III) anions), which have square planar molecular geometry.
References
- ↑ Weick, C. F.; Basolo, Fred (1966). "The Aqueous Solution Chemistry and Kinetic Behavior of a Pseudo-Octahedral Complex of Gold(III)". Inorg. Chem. 5 (4): 576. doi:10.1021/ic50038a018.
- ↑ Afanasieva, V. A.; Glinskaya, L. A.; Klevtsova, R. F.; Mironov, I. V.; Sheludyakova, L. A. (2007). "Introduction of halogen atoms into gold(III) tetraaza metallocomplexes. Crystal and molecular structure of [Au(C9H18N4Br)](ClO4)2 and [Au(C14H20N4Br2)]ClO4". J. Struc. Chem. 48 (2): 289. doi:10.1007/s10947-007-0045-5.
 |
