Chemistry:Gold(III) fluoride
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Gold(III) fluoride
| |
| Other names
Gold trifluoride
Auric fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| AuF3 | |
| Molar mass | 253.961779 g·mol−1 |
| Appearance | orange-yellow hexagonal crystals |
| Density | 6.75 g/cm3 |
| Melting point | sublimes above 300°C |
| Reacts[2][3] | |
| +74·10−6 cm3/mol | |
| Structure | |
| Hexagonal, hP24 | |
| P6122, No. 178 | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-363.3 kJ/mol |
| Related compounds | |
Other anions
|
Gold(III) chloride Gold(III) bromide |
Other cations
|
Silver fluoride Copper(II) fluoride Mercury(II) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gold(III) fluoride, AuF
3, is an orange solid that sublimes at 300 °C.[4] It is a powerful fluorinating agent. It is very sensitive to moisture, yielding gold(III) hydroxide and hydrofluoric acid.
Preparation
AuF3 can be prepared by reacting AuCl3 with F2 or BrF3.[3]
Structure
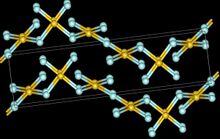
The crystal structure of AuF3 consists of spirals of square-planar AuF4 units.[5]
 |
200px | 80px | 100px | 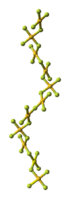
|
| AuF3 unit cell | neighbouring (AuF3)n helices | distorted octahedral coordination of gold by six fluorines | top-down view of an (AuF3)n helix | side view of an (AuF3)n helix |
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–59. ISBN 0-8493-0594-2.
- ↑ Victor Lenher (1903). "Fluoride of Gold.1" (in en). Journal of the American Chemical Society 25 (11): 1136–1138. doi:10.1021/ja02013a004. https://zenodo.org/record/1707291.
- ↑ 3.0 3.1 Inis C. Tornieporth-Oetting; Thomas M. Klapötke (1995). "Laboratory Scale Direct Synthesis of Pure AuF3" (in en). Chemische Berichte 128 (9): 957–958. doi:10.1002/cber.19951280918.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8., p. 1184.
- ↑ F. W. B. Einstein; P. R. Rao; James Trotter; Neil Bartlett (1967). "The crystal structure of gold trifluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical 4: 478–482. doi:10.1039/J19670000478.
External links
 |
