Chemistry:Gold(III) oxide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Gold(III) oxide
| |
| Other names
Gold trioxide, Gold sesquioxide, Auric oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Au2O3 | |
| Molar mass | 441.93 |
| Appearance | red-brown solid |
| Melting point | 298 °C (568 °F; 571 K)[1] |
| insoluble in water, soluble in hydrochloric and nitric acid | |
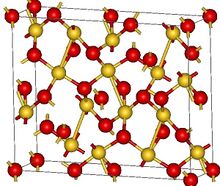
| Structure | |
| Orthorhombic, oF40 | |
| Fdd2, No. 43[2] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gold(III) oxide (Au2O3) is an inorganic compound of gold and oxygen with the formula Au2O3. It is a red-brown solid that decomposes at 298 °C.[3]
According to X-ray crystallography, Au2O3 features square planar gold centers with both 2- and 3-coordinated oxides. The four Au-O bond distances range from 193 to 207 picometers.[2] The crystals can be prepared by heating amorphous hydrated gold(III) oxide with perchloric acid and an alkali metal perchlorate in a sealed quartz tube at a temperature of around 250 °C and a pressure of around 30 MPa.[4]
References
- ↑ Kawamoto, Daisuke; Ando, Hiroaki; Ohashi, Hironori; Kobayashi, Yasuhiro; Honma, Tetsuo; Ishida, Tamao; Tokunaga, Makoto; Okaue, Yoshihiro et al. (2016-11-15). "Structure of a Gold(III) Hydroxide and Determination of Its Solubility". Bulletin of the Chemical Society of Japan (The Chemical Society of Japan) 89 (11): 1385–1390. doi:10.1246/bcsj.20160228. ISSN 0009-2673.
- ↑ 2.0 2.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedstruct - ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Jones, Peter G.; Rumpel, Horst; Sheldrick, George M.; Schwarzmann, Einhard (1980). "Gold(III) oxide and oxychloride" (open access). Gold Bulletin 13 (2): 56. doi:10.1007/BF03215453.
External links
 |


