Chemistry:Gold(I) sulfide
| |
| Names | |
|---|---|
| IUPAC name
Gold(I) sulfide
| |
| Other names
Aurous sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Au 2S | |
| Molar mass | 425.998 g/mol |
| Density | 11 g/cm3[1] |
| Melting point | 240 °C (464 °F; 513 K) |
| Insoluble | |
| Related compounds | |
Other anions
|
Copper(I) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gold(I) sulfide is the inorganic compound with the formula Au
2S. It is the principal sulfide of gold. It decomposes to gold metal and elemental sulfur, illustrating the "nobility" of gold.
Structure and preparation
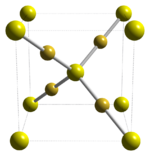
The compound crystallizes in the motif seen for cuprous oxide: gold is 2-coordinate, sulfur 4-coordinate, and the S-Au-S linkage is linear.[2] Linear coordination geometry is typical of gold(I) compounds, e.g. the coordination complex chloro(dimethyl sulfide)gold(I). The structure is similar to the α form of silver sulfide (argentite), which only exists at high temperatures.[3]
It can be prepared by treating gold chloride with hydrogen sulfide[4] It also arises by sulfiding dicyanoaurate:
This product is described as "initially dark reddish-brown" solid that turns "steel-gray".[5][3]
References
- ↑ Perry, Dale, ed (1995) (in English). Handbook of Inorganic Compounds. p. 184. ISBN 9780849386718.
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN:0-19-855370-6.
- ↑ 3.0 3.1 Ishikawa, K. (1995). "Structure and Electrical Properties of Au2S". Solid State Ionics 79: 60–66. doi:10.1016/0167-2738(95)00030-A.
- ↑ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ↑ Faltens, Marjorie O. (1970). "Mössbauer Spectroscopy of Gold Compounds". The Journal of Chemical Physics 53 (11): 4249–4264. doi:10.1063/1.1673931. Bibcode: 1970JChPh..53.4249F.
 |

