Chemistry:Tetrahydroxyborate
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetrahydroxyborate
| |||
| Systematic IUPAC name | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 1966 | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| [H4BO4]− | |||
| Molar mass | 78.840 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
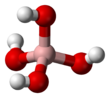
Tetrahydroxyborate is an inorganic anion with the chemical formula [BH
4O
4]−
or [B(OH)
4]−
. It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, Ca(B(OH)
4)
2 • 2H2O, originally formulated CaB
2O
4 • 6H2O.[2] It is one of the boron oxoanions, and acts as a weak base. The systematic names are tetrahydroxyboranuide (substitutive) and tetrahydroxidoborate(1−) (additive).[1] It can be viewed as the conjugate base of boric acid.
Structure
Tetrahydroxyborate has a symmetric tetrahedral geometry,[3]: p.203–205 isoelectronic with the hypothetical compound orthocarbonic acid (C(OH)
4).
Chemical properties
Basicity
Tetrahydroxyborate acts as a weak Brønsted–Lowry base because it can assimilate a proton (H+
), yielding boric acid with release of water:
- B(OH)−
4 + H+ ⇌ B(OH)
3 + H
2O
It can also release a hydroxide anion HO−
, thus acting as a classical Arrhenius base:
Thus, when boric acid is dissolved in pure (neutral) water, most of it will exist as tetrahydroxyborate ions.[citation needed]
With diols
In aqueous solution, the tetrahydroxyborate anion reacts with cis-vicinal diols (organic compounds containing similarly-oriented hydroxyl groups in adjacent carbon atoms), (R
1,R
2)=C(OH)–C(OH)=(R
3,R
4)) such as mannitol, sorbitol, glucose and glycerol, to form anion esters containing one or two five-member –B–O–C–C–O– rings.[4]
For example, the reaction with mannitol can be written as
- [B(OH)
4]−
+ H(HCOH)
6H ⇌ [B(OH)
2(H(HCOH)
2(HCO–)
2(HCOH) )]−
+ 2 H
2O - [B(OH)
2(H(HCOH)
2(HCO–)
2(HCOH)
2H)]−
+ H(HCOH)
6H ⇌ [B(H(HCOH)
2(HCO–)
2(HCOH)
2H)
2]−
+ 2 H
2O
Giving the overall reaction
- [B(OH)
4]−
+ 2 H(HCOH)
6H ⇌ [B(H(HCOH)
2(HCO–)
2(HCOH)
2H)
2]−
+ 4 H
2O
These mannitoborate esters are fairly stable and thus depletes the tetrahydroxyborate from the solution.[5][6][3]
The addition of mannitol to an initially neutral solution containing boric acid or borates lowers the pH enough for the be titrated by a strong base as NaOH, including with an automated a potentiometric titrator. This is a reliable method to assay the amount of borate content present in the solution.[3]
Other chemical reactions
Upon treatment with a strong acid, a metal tetrahydroxyborate converts to boric acid and the metal salt.
Oxidation of tetrahydroxyborate gives the perborate anion [B
2O
4(OH)
4]2−:
- 2[B(OH)
4]−
+ 2O → [B
2O
4(OH)
4]2− + 2H
2O
When heated to a high temperature, tetrahydroxyborate salts decompose to produce metaborate salts and water, or to produce boric acid and a metal hydroxide:
- n [B(OH)
4]−
→ (([BO
2]−
)
n) + 2n H
2O - [B(OH)
4]−
→ B(OH)
3 + HO−
Production
Tetrahydroxyborate salts are produced by treating boric acid with an alkali such as sodium hydroxide, with catalytic amounts of water. Other borate salts may be obtained by altering the process conditions.
Uses
Tetrahydroxyborate can be used as a cross-link in polymers.
Occurrence
The tetrahydroxyborate anion is found in Na[B(OH)4],[7] Na2[B(OH)4]Cl and CuII[B(OH)4]Cl.
-
space-filling model of the crystal
structure of sodium tetrahydroxyborate
See also
References
- ↑ 1.0 1.1 1.2 "Tetrahydroxoborate(1−) (CHEBI:41132)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=41132.
- ↑ Glossary of Geology,5th edition, 2005, ISBN 978-0922152766 ed. by Julia A. Jackson, James P. Mehl, Klaus K. E. Neuendorf, American Geological Institute
- ↑ 3.0 3.1 3.2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Lyman F. Kebler (1894): "On the interaction of borax, carbonates and polyhydric alcohols; also on the composition of borax". Journal of the Franklin Institute, volume 138, issue 3, pages 236-239. doi:10.1016/0016-0032(94)90292-5
- ↑ NIST Special Publication. U.S. Government Printing Office. 1969.
- ↑ Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, p. 357, ISBN 0-582-22628-7.
- ↑ L. J. Csetenyi; F. P. Glasser; R. A. Howie (June 1993). "Structure of sodium tetrahydroxyborate". Acta Crystallographica C 49 (6): 1039–1041. doi:10.1107/S0108270193000058.
 |