Chemistry:Tin(II) oxalate
From HandWiki
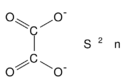
| |
| Names | |
|---|---|
| Other names
Tin(II) oxalate, Stannous oxalate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2O4Sn | |
| Molar mass | 206.728 g·mol−1 |
| Appearance | colorless crystals |
| Density | 3.56 |
| Melting point | 280 °C (536 °F; 553 K)[1] |
| 0.5 g/l | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H312, H318 | |
| P264, P270, P280, P301+312, P302+352, P305+351+338, P310, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds
|
Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Praseodymium oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tin(II) oxalate is an inorganic compound, a salt of tin and oxalic acid with the chemical formula SnC2O4.[2] The compound looks like colorless crystals, does not dissolve in water, and forms crystalline hydrates.
Synthesis
Effect of oxalic acid solution on tin(II) oxide :
- [math]\displaystyle{ \mathsf{ SnO + H_2C_2O_4 \ \xrightarrow{}\ SnC_2O_4\downarrow + H_2O } }[/math]
Tin(II) oxalate can also be obtained by using tin(II) chloride and oxalic acid.[3]
Properties
Tin (II) oxalate forms colorless crystals.
Insoluble in water and acetone. Soluble in dilute HCl,[4] methanol, and petroleum ether.[5]
Forms crystal hydrates of the composition SnC2O4•n H2O, where n = 1 and 2.
Decomposes on heating:
- [math]\displaystyle{ \mathsf{ SnC_2O_4 \ \xrightarrow{380^oC}\ SnO_2 + 2CO } }[/math]
Applications
- Tin oxalate is used as a catalyst in the production of organic esters and plasticizers.[4]
- It is used for dyeing and printing fabrics.
- The compound is also used in stannous oral care compositions.
- Few studies have reported on the use of tin(II) oxalate as an anode material for rechargeable lithium batteries.[6]
References
- ↑ "Tin Oxalate" (in en). American Elements. https://www.americanelements.com/tin-oxalate-814-94-8.
- ↑ "Tin(II) oxalate 98% | Sigma-Aldrich" (in en). sigmaaldrich.com. https://www.sigmaaldrich.com/RU/ru/product/ALDRICH/402761.
- ↑ Nagirnyak, Svitlana V.; Lutz, Victoriya A.; Dontsova, Tatiana A.; Astrelin, Igor M. (26 July 2016). "Synthesis and Characterization of Tin(IV) Oxide Obtained by Chemical Vapor Deposition Method". Nanoscale Research Letters 11 (1): 343. doi:10.1186/s11671-016-1547-x. ISSN 1556-276X. PMID 27456501. Bibcode: 2016NRL....11..343N.
- ↑ 4.0 4.1 "814-94-8 - Tin(II) oxalate - Stannous oxalate - 14113 - Alfa Aesar". Alfa Aesar. https://www.alfa.com/ru/catalog/014113/.
- ↑ "Registration Dossier - ECHA". European Chemical Agency. https://echa.europa.eu/registration-dossier/-/registered-dossier/11280/4/10.
- ↑ Park, Jae-Sang; Jo, Jae-Hyeon; Yashiro, Hitoshi; Kim, Sung-Soo; Kim, Sun-Jae; Sun, Yang-Kook; Myung, Seung-Taek (9 August 2017). "Synthesis and Electrochemical Reaction of Tin Oxalate-Reduced Graphene Oxide Composite Anode for Rechargeable Lithium Batteries". ACS Applied Materials & Interfaces 9 (31): 25941–25951. doi:10.1021/acsami.7b03325. ISSN 1944-8252. PMID 28718628. https://pubmed.ncbi.nlm.nih.gov/28718628/. Retrieved 5 August 2021.
 |

