Chemistry:Tin(II) sulfate
From HandWiki
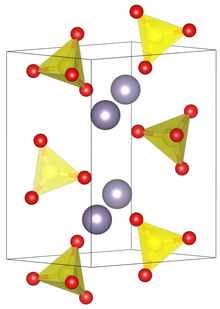 Unit cell of tin(II) sulfate.
| |
| Names | |
|---|---|
| Other names
Stannous sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| SnSO4 | |
| Molar mass | 214.773 g/mol |
| Appearance | white-yellowish crystalline solid deliquescent |
| Density | 4.15 g/cm3 |
| Melting point | 378 °C (712 °F; 651 K) |
| Boiling point | decomposes to SnO2 and SO2 |
| 33 g/100 mL (25 °C) | |
| Structure[1] | |
| Primitive orthorhombic | |
| Pnma, No. 62 | |
a = 8.80 Å, b = 5.32 Å, c = 7.12 Å[1]
| |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2207 mg/kg (oral, rat) 2152 mg/kg (oral, mouse)[2] |
| Related compounds | |
Other anions
|
Tin(II) chloride, tin(II) bromide, tin(II) iodide |
Other cations
|
Lead(II) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tin(II) sulfate (SnSO4) is a chemical compound. It is a white solid that can absorb enough moisture from the air to become fully dissolved, forming an aqueous solution; this property is known as deliquescence. It can be prepared by a displacement reaction between metallic tin and copper(II) sulfate:[3]
- Sn (s) + CuSO4 (aq) → Cu (s) + SnSO4 (aq)
Tin(II) sulfate is a convenient source of tin(II) ions uncontaminated by tin(IV) species.
Structure
In the solid state the sulfate ions are linked together by O-Sn-O bridges. The tin atom has three oxygen atoms arranged pyramidally at 226 pm with the three O-Sn-O bond angles of 79°, 77.1° and 77.1°. Other Sn-O distances are longer ranging from 295 - 334pm.[3][4]
References
- ↑ 1.0 1.1 Donaldson, J. D.; Puxley, D. C. (1972). "The crystal structure of tin(II) sulphate". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 28 (3): 864–867. doi:10.1107/S0567740872003322.
- ↑ "Tin (inorganic compounds, as Sn)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7440315.html.
- ↑ 3.0 3.1 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 451. ISBN 978-0-08-022057-4. https://books.google.com/books?id=OezvAAAAMAAJ&q=0-08-022057-6&dq=0-08-022057-6&source=bl&ots=m4tIRxdwSk&sig=XQTTjw5EN9n5z62JB3d0vaUEn0Y&hl=en&sa=X&ei=UoAWUN7-EM6ziQfyxIDoCQ&ved=0CD8Q6AEwBA.
- ↑ Donaldson, J. D.; Puxley, D. C. (1972). "The crystal structure of tin(II) sulphate". Acta Crystallographica Section B 28 (3): 864–867. doi:10.1107/S0567740872003322. ISSN 0567-7408.
 |


