Chemistry:Vanadium(III) acetylacetonate
From HandWiki
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C15H21O6V | |
| Molar mass | 348.269 g·mol−1 |
| Appearance | Brown solid |
| Density | 1.334 g/cm3 |
| Melting point | 184 °C (363 °F; 457 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H311, H315, H318, H319, H330, H335 | |
| P260, P261, P264, P270, P271, P280, P284, P301+310, P302+352, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P332+313, P337+313, P361, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
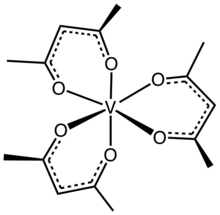
Vanadium(III) acetylacetonate is the coordination compound with the formula V(C5H7O2)3, sometimes designated as V(acac)3. It is an orange-brown solid that is soluble in organic solvents.
Structure and synthesis
The complex has idealized D3 symmetry.[1] Like other V(III) compounds, it has a triplet ground state.
The compound is prepared by reduction of ammonium vanadate in the presence of acetylacetone.[2]
Applications and research
V(acac)3 is a common precatalyst for the production of EPDM polymers.[3]
It has also been shown to be a precursor to vanadium pentoxide nanostructures.[4]
References
- ↑ C. A. L. Filgueiras; A. Horn Jr.; R. A. Howie; J. M. S. Skakle; J. L. Wardell (2001). "α-Form of tris(2,4-pentanedionato-O,O')vanadium(III), re-refinement against new intensity data". Acta Crystallogr. E 57: m157–m158. doi:10.1107/S1600536801004391.
- ↑ S. Dilli; E. Patsalides (1976). "A convenient new Method for the preparation of vanadium(III) β-diketonates". Australian Journal of Chemistry 29 (11): 2389–2393. doi:10.1071/CH9762389.
- ↑ Ma, Yinlin; Reardon, Damien; Gambarotta, Sandro; Yap, Glenn; Zahalka, Hayder; Lemay, Catherine (1999). "Vanadium-Catalyzed Ethylene-Propylene Copolymerization: The Question of the Metal Oxidation State in Ziegler-Natta Polymerization Promoted by (β-diketonate)3V". Organometallics 18: 2773–2781. doi:10.1021/om9808763.
- ↑ Cao, An-Min; Hu, Jin-Song; Liang, Han-Pu; Wan, Li-Jun (2005). "Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries". Angewandte Chemie International Edition 44 (28): 4391–4395. doi:10.1002/anie.200500946. PMID 15942965.
 |

