Chemistry:Xanthohumol
| |
| Names | |
|---|---|
| Preferred IUPAC name
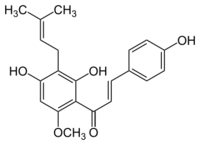
2′,4,4′-Trihydroxy-6′-methoxy-3′-(3-methylbut-2-en-1-yl)chalcone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C21H22O5 | |
| Molar mass | 354.402 g·mol−1 |
| Density | 1.24 g/cm3[1] |
| Melting point | 157–159 °C (315–318 °F; 430–432 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops.[2] Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.[3][4]
Biosynthesis
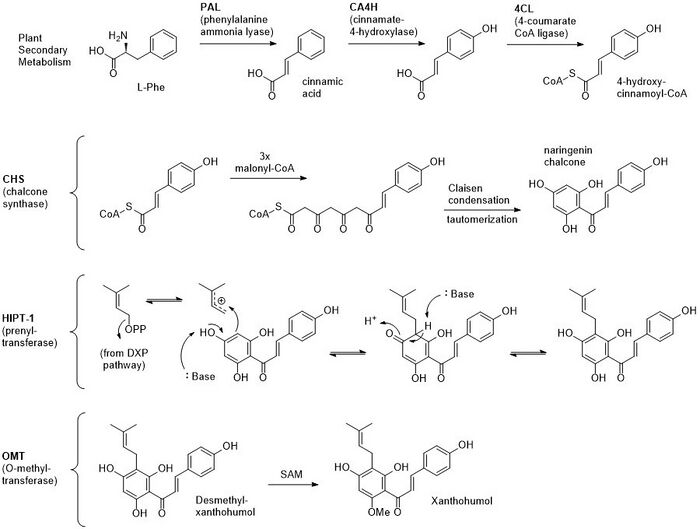
Xanthohumol is a prenylated chalconoid derived from a plant type III PKS, and is synthesized in the glandular trichromes of hop cones.[2] L-Phenylalanine serves as the starting material, which is converted to cinnamic acid by the PLP-dependent phenylalanine ammonia lyase.[5][6] Cinnamic acid is oxidized by cinnamate-4-hydroxylase and loaded onto Coenzyme A (CoA) by 4-coumarate CoA ligase to yield 4-hydroxy-cinnamoyl CoA, the starter unit for PKS extension.[5][6] This molecule is extended three times with malonyl CoA, cyclized through a Claisen condensation, and aromatized through tautomerization to form naringenin chalcone (chalconaringenin).[5] This intermediate has the potential to form a variety of different products depending on the enzymes that modify the core structure.[2][5] In the case of xanthohumol, a prenyltransferase called Humulus lupulus prenyltransferase 1 (HlPT-1) attaches a molecule of dimethylallyl pyrophosphate from the DXP pathway.[7] HlPT-1 has a broad substrate specificity and also participates in making other prenylated flavonoids in the hop plant.[7] Finally, an O-methyltransferase methylates a phenol substituent using S-adenosyl methionine.[6] Total syntheses of xanthohumol and derivatives have been achieved, though extraction from hops remains a primary source.[8][9]
Beer
In commercial beers, the concentration of xanthohumol ranges from about 2 μg/L – 1.2 mg/L.[10] During the brewing process, xanthohumol and other prenylated flavonoids are lost as they are converted to the corresponding flavanones.[11] Different hop varieties and different beers contain varying quantities of xanthohumol.[2]
Research
Xanthohumol is under basic research for its potential biological properties.[10] Xanthohumol can be extracted with pressurized hot water.[12]
See also
- Isoxanthohumol, the corresponding prenylated flavanone
- 8-Prenylnaringenin, a related prenylflavanoid with anti-estrogenic activity
- Alpha acids, a class of bitter compounds in hops
- Myrcene, humulene, and caryophyllene, essential oils in hops
References
- ↑ 1.0 1.1 Xanthohumol from hop (Humulus lupus), Santa Cruz Biotechnology
- ↑ 2.0 2.1 2.2 2.3 Stevens, Jan F.; Page, Jonathan E. (May 2004). "Xanthohumol and related prenylflavonoids from hops and beer: to your good health!". Phytochemistry 65 (10): 1317–1330. doi:10.1016/j.phytochem.2004.04.025. ISSN 0031-9422. PMID 15231405.
- ↑ Goese, Markus; Kammhuber, Klaus; Bacher, Adelbert; Zenk, Meinhart H.; Eisenreich, Wolfgang (1999-07-15). "Biosynthesis of bitter acids in hops" (in en). European Journal of Biochemistry 263 (2): 447–454. doi:10.1046/j.1432-1327.1999.00518.x. ISSN 1432-1033. PMID 10406953.
- ↑ Wang, Guodong; Tian, Li; Aziz, Naveed; Broun, Pierre; Dai, Xinbin; He, Ji; King, Andrew; Zhao, Patrick X. et al. (November 2008). "Terpene biosynthesis in glandular trichomes of hop". Plant Physiology 148 (3): 1254–1266. doi:10.1104/pp.108.125187. ISSN 0032-0889. PMID 18775972.
- ↑ 5.0 5.1 5.2 5.3 M., Dewick, Paul. Medicinal natural products : a biosynthetic approach. ISBN 9780470741689. OCLC 259265604.
- ↑ 6.0 6.1 6.2 Nagel, Jana; Culley, Lana K.; Lu, Yuping; Liu, Enwu; Matthews, Paul D.; Stevens, Jan F.; Page, Jonathan E. (January 2008). "EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol". The Plant Cell 20 (1): 186–200. doi:10.1105/tpc.107.055178. ISSN 1040-4651. PMID 18223037.
- ↑ 7.0 7.1 Tsurumaru, Yusuke; Sasaki, Kanako; Miyawaki, Tatsuya; Uto, Yoshihiro; Momma, Takayuki; Umemoto, Naoyuki; Momose, Masaki; Yazaki, Kazufumi (2012-01-06). "HlPT-1, a membrane-bound prenyltransferase responsible for the biosynthesis of bitter acids in hops". Biochemical and Biophysical Research Communications 417 (1): 393–398. doi:10.1016/j.bbrc.2011.11.125. ISSN 1090-2104. PMID 22166201.
- ↑ Khupse, Rahul S.; Erhardt, Paul W. (2007-09-01). "Total Synthesis of Xanthohumol". Journal of Natural Products 70 (9): 1507–1509. doi:10.1021/np070158y. ISSN 0163-3864. PMID 17844997. https://figshare.com/articles/Total_Synthesis_of_Xanthohumol/2983579.
- ↑ Zhang, Baoxin; Duan, Dongzhu; Ge, Chunpo; Yao, Juan; Liu, Yaping; Li, Xinming; Fang, Jianguo (2015-02-26). "Synthesis of Xanthohumol Analogues and Discovery of Potent Thioredoxin Reductase Inhibitor as Potential Anticancer Agent". Journal of Medicinal Chemistry 58 (4): 1795–1805. doi:10.1021/jm5016507. ISSN 0022-2623. PMID 25629304.
- ↑ 10.0 10.1 Gerhäuser, Clarissa (September 2005). "Beer constituents as potential cancer chemopreventive agents". European Journal of Cancer 41 (13): 1941–1954. doi:10.1016/j.ejca.2005.04.012. ISSN 0959-8049. PMID 15953717.
- ↑ Stevens, Jan F.; Taylor, Alan W.; Clawson, Jeff E.; Deinzer, Max L. (1999-06-01). "Fate of Xanthohumol and Related Prenylflavonoids from Hops to Beer". Journal of Agricultural and Food Chemistry 47 (6): 2421–2428. doi:10.1021/jf990101k. ISSN 0021-8561. PMID 10794646.
- ↑ A. Gil-Ramírez, J.A. Mendiola, E. Arranz, A. Ruíz-Rodríguez, G. Reglero, E. Ibáñez, F.R. Marín. Highly isoxanthohumol enriched hop extract obtained by pressurized hot water extraction (PHWE). Chemical and functional characterization. Innovative Food Science and Emerging Technologies 2012 October, vol 16 Pages 54-60. doi: 10.1016/j.ifset.2012.04.006.
