Chemistry:Zhan catalyst
| |
| Names | |
|---|---|
| IUPAC name
Dichloro(1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)((5-((dimethylamino)sulfonyl)-2-(1-methylethoxy-O)phenyl)methylene-C)ruthenium(II)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C33H43Cl2N3O3RuS | |
| Molar mass | 733.75 g·mol−1 |
| Appearance | Green solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
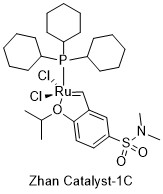
| |
| Names | |
|---|---|
| IUPAC name
Dichloro((5-((dimethylamino)sulfonyl)-2-(1-methylethoxy-O)phenyl)methylene-C)(tricyclohexylphosphine)ruthenium(IV)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C30H50Cl2NO3PRuS | |
| Molar mass | 707.74 g·mol−1 |
| Appearance | Brown solid |
| Melting point | 145 to 155 °C (293 to 311 °F; 418 to 428 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A Zhan catalyst is a type of ruthenium-based organometallic complex used in olefin metathesis. This class of chemicals is named after the chemist who first synthesized them, Zheng-Yun J. Zhan.[1]
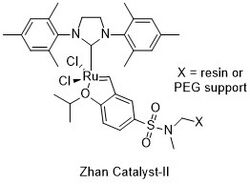
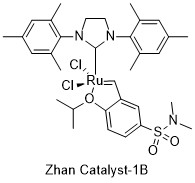
These catalysts are ruthenium complexes with functionally substituted alkoxybenzylidene carbene ligands, which can be chemically bonded to the surface of resins, PEG chains, and polymers. Like the structurally similar Hoveyda-Grubbs catalyst, they contain an isopropoxystyrene moiety, but include an extra electron-withdrawing sulfonamide group attached to the carbon para to the phenol oxygen. Of the three catalysts, Zhan Catalyst-1B and -1C both contain a dimethylsulfonamide moiety attached to the aryl ring, while Zhan Catalyst-II is connected to a resin via a sulfonamide linker.[2][3][4]
History
The Zhan catalysts were inspired by previous work in the olefin metathesis field. Robert H. Grubbs first reported the first and second generation of Ru catalysts in 1992, with good metathesis activity. However, the catalysts containing the tricyclohexylphospine ligand were unstable to air and water, and the catalytic activity is not good enough for some multiple substituted olefin substrates.[5][6][7][8]
In 1999, Amir H. Hoveyda showed that alkoxybenzylidene ligand based Ru catalysts offered higher activity and better stability than their Grubbs counterparts without these ligands.[9][10] Later, Grela (2002) and Blechert (2003) further improved catalyst activity by incorporating substitution to Hoveyda’s alkoxybenzylidene ligands.[11][12] Zhan’s catalysts were first reported in 2007, and include electron-withdrawing groups like dimethylsulfonamide on the aryl ring. Zhan's second generation catalysts are also tethered to a resin or PEG-linked support via the sulfonamide group on the isopropoxystyrene.[1]
As with other Grubbs-type catalysts with modified chelating benzylidenes, after one catalytic turnover, the chelate is no longer associated with the propagating catalyst, meaning that the initiate rate, the rate of o-alkoxystyrene rechelation, and the rates of various catalyst decomposition events are the factors that differ between the Zhan catalysts and the parent Hoveyda–Grubbs catalysts. A mechanistic study by Plenio and coworkers in 2012 suggested that the Zhan compounds, like other Hoveyda-type catalysts, initiate by competing dissociative and interchange mechanisms, with the relative activation energies being a function of catalyst structure, olefin identity, and reaction conditions.[13] However, nobody had been able to rigorously establish through experimentation how the various changes to the structure affected catalytic activity of the complex. Engle, Luo, Houk, Grubbs, and coworkers developed a model that could rationalize initiation rates of ruthenium olefin metathesis catalysts with chelated benzylidenes, using a combination of organometallic synthesis, reaction kinetics, NMR spectroscopy, X-ray crystallography, and DFT calculations.[14]
Preparation
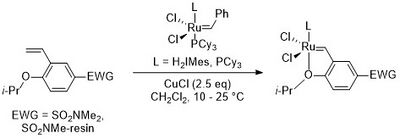
In order to make the catalysts, the pre-complex is treated with CuCl and the isopropoxystyrene ligand.[1]
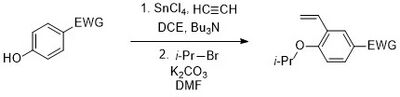
The isopropoxystyrene ligand is prepared using an ortho-vinylation of the phenol with ethyne, using conditions first proposed by Masahiko Yamaguchi in 1998. Here, SnCl4 and Bu3N were added to ethyne to generate stannylacetylene, which is the active vinylating species in this C–C bond formation.[15] After coupling, the phenol can be alkylated using i-PrBr and a base.
Applications
Unlike the Grubbs or Hoveyda-Grubbs catalysts, the Zhan catalysts can be recovered and recycled by simple precipitation or filtration. Zhan Catalyst-1B and -1C are soluble in dichloromethane, dichloroethane, chloroform, ether, and other solvents, but insoluble in methanol, ethanol, and other alcohols. Zhan Catalyst-II is linked to a resin- and PEG-linked support, offering a great advantage in recyclable utility, and leaving little or no trace of toxic metal contamination within the product of olefin metathesis reactions. These catalysts can then be reused again, allowing for broad application in the chemical and pharmaceutical industries.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Zhan, Zheng-Yun. "Recyclable ruthenium catalysts for metathesis reactions". United States Patent and Trademark Office. https://www.google.com/patents/US20070043180.
- ↑ "Zhan Catalyst-1B". Strem Chemicals Product Catalog. https://www.strem.com/catalog/v/44-0082/59/ruthenium_918870-76-5.
- ↑ "Zhan Catalyst-1C". Strem Chemicals Product Catalog. https://www.strem.com/catalog/v/44-0078/59/ruthenium_918871-44-0.
- ↑ "Zhan Catalyst-II". Strem Chemicals Product Catalog. https://www.strem.com/catalog/v/44-0083/59/ruthenium.
- ↑ Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. (1992). "Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media". J. Am. Chem. Soc. 114 (10): 3974–3975. doi:10.1021/ja00036a053. https://authors.library.caltech.edu/88217/2/ja00036a053_si_001.pdf.
- ↑ Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. (1999). "Synthesis and activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands". Org. Lett. 1 (6): 953–956. doi:10.1021/ol990909q. PMID 10823227.
- ↑ Grubbs, Robert H.; Sonbinh T. Nguyen. "High activity ruthenium or osmium metal carbene complexes for olefin metathesis reactions and synthesis thereof". World Intellectual Property Organization. https://www.google.com/patents/WO1996004289A1?cl=en.
- ↑ Grubbs, Robert H.; Matthias Scholl. "Imidazolidine-based metal carbene metathesis catalysts". World Intellectual Property Organization. https://www.google.com/patents/WO2000071554A2?cl=en28.
- ↑ Kingsbury, J. S.; Harrity, J. P. A.; Bonitatebus, P. J.; Hoveyda, A. H. (1999). "A Recyclable Ru-Based Metathesis Catalyst". J. Am. Chem. Soc. 121 (4): 791–799. doi:10.1021/ja983222u.
- ↑ Hoveyda, Amir H.; Jason Kingsbury. "Recyclable metathesis catalysts". United States Patent and Trademark Office. https://www.google.com/patents/US20020107138.
- ↑ Grela, K.; Harutyunyan, S.; Michrowska, A. (2002). "A Highly Efficient Ruthenium Catalyst for Metathesis Reactions". Angew. Chem. Int. Ed. 41 (21): 4038–4040. doi:10.1002/1521-3773(20021104)41:21<4038::AID-ANIE4038>3.0.CO;2-0. PMID 12412074. https://pure.rug.nl/ws/files/3040319/2002AngewChemIntEdGrela.pdf.
- ↑ Blechert, Siegfried. "Novel transition metal complexes and their use in transition metal-catalysed reactions". United States Patent and Trademark Office. https://www.google.com/patents/US20030220512.
- ↑ Thiel, V.; Hendann, M.; Wannowius, K-J.; Plenio, H. (2012). "On the mechanism of the initiation reaction in Grubbs-Hoveyda complexes". J. Am. Chem. Soc. 134 (2): 1104–1114. doi:10.1021/ja208967h. PMID 22188483.
- ↑ Engle, K. M.; Lu, G.; Luo, S-X.; Henling, L. M.; Takase, M. K.; Liu, P.; Houk, K. N.; Grubbs, R. H. (2015). "Origins of Initiation Rate Differences in Ruthenium Olefin Metathesis Catalysts Containing Chelating Benzylidenes". J. Am. Chem. Soc. 137 (17): 5782–5792. doi:10.1021/jacs.5b01144. PMID 25897653.
- ↑ Yamaguchi, M.; Arisawa, M.; Omata, K.; Kuninobu, K.; Hirama, M.; Uchimaru, T. (1998). "Ortho-Vinylation Reaction of Phenols with Ethyne". J. Org. Chem. 63 (21): 7298–7305. doi:10.1021/jo980785f. PMID 11672375.
 |