Chemistry:Zinc azide
| |
| Names | |
|---|---|
| IUPAC name
Zinc(II) azide
| |
| Other names
Zinc diazide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Zn(N 3) 2 | |
| Molar mass | 149.4 g/mol |
| Appearance | White solid |
| Density | 2.559 g/cm3 (α polymorph) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc azide Zn(N
3)
2 is an inorganic compound composed of zinc cations (Zn2+) and azide anions (N−
3). It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:[1]
- Zn(C
2H
5)
2 + 2 HN
3 → Zn(N
3)
2 + 2 C
2H
6
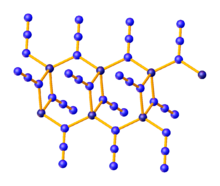
Properties
Zinc azide is a coordination polymer which crystallizes in three polymorphs, all of which feature tetrahedral zinc centers and bridging azide ligands. α-Zn(N
3)
2 crystallizes in the monoclinic space group and is stable, while the other two polymorphs are metastable. P21/n. β-Zn(N
3)
2 is trigonal, space group P3221, and γ-Zn(N
3)
2 is monoclinic, space group C2.
It is easily hydrolyzed, and attempts to prepare it in aqueous solution resulted in the precipitation of basic azides Zn(OH)
2-x(N
3)
x (x = 0.9–1.0). Both the α- and β-forms were found to be very friction- and shock-sensitive, violently exploding in blue flashes, but can be made to decompose slowly by gentle heating, giving off nitrogen gas. In a sealed glass tube with inert atmosphere, this yields zinc nitride, Zn
3N
2.[1]
References
 |
