Chemistry:Zinc iodide
| |
| Names | |
|---|---|
| IUPAC name
Zinc iodide
| |
| Other names
Zinc(II) iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| ZnI2 | |
| Molar mass | 319.19 g/mol |
| Appearance | white solid |
| Density | 4.74 g/cm3 |
| Melting point | 446 °C (835 °F; 719 K) |
| Boiling point | 1,150 °C (2,100 °F; 1,420 K) decomposes |
| 450 g/100mL (20 °C) | |
| −98.0·10−6 cm3/mol | |
| Structure | |
| Tetragonal, tI96 | |
| I41/acd, No. 142 | |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | 625 °C (1,157 °F; 898 K) |
| Related compounds | |
Other anions
|
Zinc fluoride Zinc chloride Zinc bromide |
Other cations
|
Cadmium iodide Mercury(I) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application.
Preparation
It can be prepared by the direct reaction of zinc and iodine in water[1][2] or refluxing ether:[3]
- Zn + I2 → ZnI2
Absent a solvent, the elements do not combine directly at room temperature.[4]
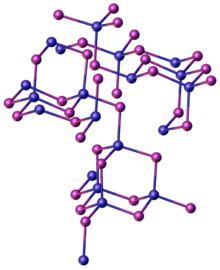
Structure as solid, gas, and in solution
The structure of solid ZnI2 is unusual relative to the dichloride. While zinc centers are tetrahedrally coordinated, as in ZnCl2, groups of four of these tetrahedra share three vertices to form “super-tetrahedra” of composition {Zn4I10}, which are linked by their vertices to form a three-dimensional structure.[5] These "super-tetrahedra" are similar to the P4O10 structure.[5][6]
Molecular ZnI2 is linear as predicted by VSEPR theory with a Zn-I bond length of 238 pm.[5]
In aqueous solution the following have been detected: Zn(H2O)62+, [ZnI(H2O)5]+, tetrahedral ZnI2(H2O)2, ZnI3(H2O)−, and ZnI42−.[7]
Applications
- Zinc iodide is often used as an x-ray opaque penetrant in industrial radiography to improve the contrast between the damage and intact composite.[8][9]
- United States patent 4,109,065 [10] describes a rechargeable aqueous zinc-halogen cell that includes an aqueous electrolytic solution containing a zinc salt selected from the class consisting of zinc bromide, zinc iodide, and mixtures thereof, in both positive and negative electrode compartments.
- In combination with osmium tetroxide, ZnI2 is used as a stain in electron microscopy.[11]
- As a Lewis acid, zinc iodide catalyzes for the conversion of methanol to triptane and hexamethylbenzene.[12]
- It can be used as a topical antiseptic.[13]
References
- ↑ F. Wagenknecht; R. Juza (1963). "Zinc iodide". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 1073.
- ↑ DeMeo, S. (1995). "Synthesis and Decomposition of Zinc Iodide: Model Reactions for Investigating Chemical Change in the Introductory Laboratory". Journal of Chemical Education 72 (9): 836. doi:10.1021/ed072p836. Bibcode: 1995JChEd..72..836D. https://pubs.acs.org/doi/abs/10.1021/ed072p836.
- ↑ Eagleson, M. (1994). Concise Encyclopedia Chemistry. Walter de Gruyter. ISBN 3-11-011451-8. https://archive.org/details/conciseencyclope00eagl.
- ↑ Gilbert, George; Houston, Kelly; Jacobsen, Jerrold J.; Phillips, David (2022) [6 Mar 2012]. Zinc iodine reaction (web video). American Chemical Society, Division of Chemical Education – via ChemEdX.
- ↑ 5.0 5.1 5.2 Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford Science Publications. ISBN 0-19-855370-6.
- ↑ Fourcroy, P. H.; Carré, D.; Rivet, J. (1978). "Structure Cristalline de l'Iodure de Zinc ZnI2". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 34 (11): 3160–3162. doi:10.1107/S0567740878010390. Bibcode: 1978AcCrB..34.3160F.
- ↑ Wakita, H.; Johansson, G.; Sandström, M.; Goggin, P. L.; Ohtaki, H. (1991). "Structure determination of zinc iodide complexes formed in aqueous solution". Journal of Solution Chemistry 20 (7): 643–668. doi:10.1007/BF00650714.
- ↑ Composite Materials for Aircraft Structures (2nd ed.). AIAA (American Institute of Aeronautics & Astronautics). 2004. ISBN 1-56347-540-5.
- ↑ Ezrin, M. (1996). Plastics Failure Guide. Hanser Gardner Publications. ISBN 1-56990-184-8. https://books.google.com/books?id=baWyaC3w3hcC.
- ↑ Will, F. G.; Secor, F. W., "Rechargeable aqueous zinc-halogen cell", US patent 4109065, issued 1978-08-22, assigned to General Electric
- ↑ Hayat, M. A. (2000). Principles and Techniques of Electron Microscopy: Biological Applications (4th ed.). Cambridge University Press. ISBN 0-521-63287-0.
- ↑ Bercaw, John E.; Diaconescu, Paula L.; Grubbs, Robert H.; Kay, Richard D.; Kitching, Sarah; Labinger, Jay A.; Li, Xingwei; Mehrkhodavandi, Parisa et al. (2006-11-01). "On the Mechanism of the Conversion of Methanol to 2,2,3-Trimethylbutane (Triptane) over Zinc Iodide". The Journal of Organic Chemistry 71 (23): 8907–8917. doi:10.1021/jo0617823. ISSN 0022-3263. PMID 17081022. https://resolver.caltech.edu/CaltechAUTHORS:20170427-090114775.
- ↑ Rohe, Dieter M. M.; Wolf, Hans Uwe (2007), "Zinc Compounds", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 1–6, doi:10.1002/14356007.a28_537, ISBN 978-3527306732
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |
