Chemistry:Zinc molybdate
From HandWiki
| |
| Identifiers | |
|---|---|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| ZnMoO4 | |
| Molar mass | 225.33 g/mol |
| Appearance | white tetragonal crystals |
| Density | 4.32 g/cm3[2] |
| Melting point | 900 °C (1,650 °F; 1,170 K) |
| insoluble | |
| Structure | |
| tetragonal | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc molybdate is an inorganic compound with the formula ZnMoO4. It is used as a white pigment, which that is also a corrosion inhibitor. A related pigment is sodium zinc molybdate, Na2Zn(MoO4)2.[4] The material has also been investigated as an electrode material.[5]
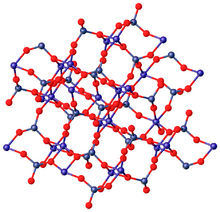
In terms of its structure, the Mo(VI) centers are tetrahedral and the Zn(II) centers are octahedral.[2]
Safety
The LD50 (oral, rats) is 11,500 mg/kg.[4] While highly soluble molybdates like e.g. sodium molybdate are toxic in higher doses, zinc molybdate is essentially non-toxic because of its insolubility in water. Molybdates possess a lower toxicity than chromates or lead salts and are therefore seen as an alternative to these salts for corrosion inhibition.
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–95, ISBN 978-0-8493-0594-8
- ↑ 2.0 2.1 Ait Ahsaine, H.; Zbair, M.; Ezahri, M.; Benlhachemi, A.; Arab, M.; Bakiz, B.; Guinneton, F.; Gavarri, J. R. (2015). "Rietveld Refinements, Impedance Spectroscopy and Phase Transition of the Polycrystalline ZnMoO4 Ceramics". Ceramics International 41 (10): 15193–15201. doi:10.1016/j.ceramint.2015.08.094. https://hal.archives-ouvertes.fr/hal-01872783/file/aitahsaine2015-1.pdf.
- ↑ "C&L Inventory". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/49701.
- ↑ 4.0 4.1 G. Etzrodt (2012). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.n20_n04.
- ↑ Hu, Xianluo; Zhang, Wei; Liu, Xiaoxiao; Mei, Yueni; Huang, Yunhui (2015). "Nanostructured Mo-based electrode materials for electrochemical Energy Storage". Chemical Society Reviews 44 (8): 2376–404. doi:10.1039/C4CS00350K. PMID 25688809.
External links
 |

