Chemistry:Zinc selenide
| |

| |
| Names | |
|---|---|
| Other names
Zinc selenide
Stilleite | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| ZnSe | |
| Molar mass | 144.35 g/mol |
| Appearance | light yellow solid |
| Density | 5.27 g/cm3 |
| Melting point | 1,525 °C (2,777 °F) |
| negligible | |
| Band gap | 2.82 eV (10 K) |
Refractive index (nD)
|
2.67 (550 nm) 2.40 (10.6 μm) |
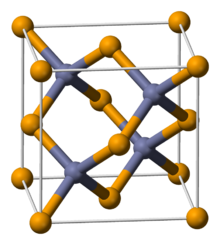
| Structure | |
| Zincblende (cubic) | |
a = 566.8 pm
| |
| Tetrahedral (Zn2+) Tetrahedral (Se2−) | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−177.6 kJ/mol |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+310, P304+340, P311, P314, P321, P330, P391, P403+233, P405, P501 | |
| Related compounds | |
Other anions
|
Zinc oxide Zinc sulfide Zinc telluride |
Other cations
|
Cadmium selenide Mercury selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F). ZnSe occurs as the rare mineral stilleite, named after Hans Stille.
Synthesis and properties
ZnSe is available in both hexagonal (wurtzite) and cubic (zincblende) polymorphs. In both cases, the Zn2+ and Se2- sites are tetrahedral. The difference in the structures related to close packing motifs, hexagonal vs cubic.
Cubic ZnSe is produced by treatment of an aqueous solution of zinc sulfate with hydrogen selenide:[1]
- ZnSO
4 + H
2Se → ZnSe + H
2SO
4
Heating the cubic form gives hexagonal ZnSe.
An alternative synthesis involves heating a mixture of zinc oxide, zinc sulfide, and selenium:
- 2 ZnO + ZnS + 3 Se → 3 ZnSe + SO
2
It is a wide-bandgap semiconductor of the II-VI semiconductor group (since zinc and selenium belong to the 12th and 16th groups of the periodic table, respectively). The material can be n-type doped with, for instance, halogen elements. P-type doping is more difficult, but can be achieved by introducing gallium.
Applications
- ZnSe is used to form II-VI light-emitting diodes and diode lasers. It emits blue light[dubious ].
- ZnSe doped with chromium (ZnSe:Cr) has been used as an infrared laser gain medium emitting at about 2.4 μm.[2]
- It is used as an infrared optical material with a remarkably wide transmission wavelength range (0.45 μm to 21.5 μm[3]). The refractive index is about 2.67 at 550 nm (green), and about 2.40 at 10.6 μm (LWIR). Similar to zinc sulfide, ZnSe is produced as microcrystalline sheets by synthesis from hydrogen selenide gas and zinc vapour. Another method of producing is a growth from melt under excessive pressure of inert gas (Ar usually).[4] When free of absorption and inclusions it is ideally suited for CO2 laser optics at 10.6 μm wavelength. It is thus a very important IR material. In daily life, it can be found as the entrance optic in the new range of "in-ear" clinical thermometers, seen as a small yellow window. Zinc selenide can slowly react with atmospheric moisture if poorly polished, but this is not generally a serious problem. Except where optics are used in spectroscopy or at the Brewster angle, antireflection or beamsplitting optical coatings are generally employed.
- ZnSe activated with tellurium (ZnSe(Te)) is a scintillator with emission peak at 640 nm, suitable for matching with photodiodes. It is used in x-ray and gamma ray detectors. ZnSe scintillators are significantly different from the ZnS ones.
Reactions
ZnSe is insoluble in water, but dissolves in concentrated hydrochloric acid.
It can be deposited as a thin film by chemical vapour deposition techniques including MOVPE and vacuum evaporation.
References
- ↑ F. Wagenknecht; R. Juza (1963). "Zinc (II) Selenide". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 2pages=1078. NY, NY: Academic Press.
- ↑ Cr2+ excitation levels in ZnSe and ZnS, G. Grebe, G. Roussos and H.-J. Schulz, J. Phys. C: Solid State Phys. vol. 9 pp. 4511-4516 (1976) doi:10.1088/0022-3719/9/24/020
- ↑ https://web.archive.org/web/20190422005411/http://www.kayelaby.npl.co.uk/general_physics/2_5/2_5_8.html Kaye and Laby online at NPL via archive.org
- ↑ "Institute for Single Crystals - Materials and Products - AIIBVI - Passive Laser Optics Elements". http://iscrystals.com/page-details.html?cat_id=78&id=91.
External links
- II-VI - INFRARED optical data & more
- University of Reading, Infrared Multilayer Laboratory optical data
 |

