Earth:Atmospheric methane

Atmospheric methane is the methane present in Earth's atmosphere.[1] The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change.[2][3] Methane is one of the most potent greenhouse gases.[4]: 82 Methane's radiative forcing (RF) of climate is direct,[5]: 2 and it is the second largest contributor to human-caused climate forcing in the historical period.[5]: 2 Methane is a major source of water vapour in the stratosphere through oxidation;[6] and water vapour adds about 15% to methane's radiative forcing effect.[7] The global warming potential (GWP) for methane is about 84 in terms of its impact over a 20-year timeframe.[8][9] That means it traps 84 times more heat per mass unit than carbon dioxide (CO2) and 105 times the effect when accounting for aerosol interactions.[10]
Since the beginning of the Industrial Revolution (around 1750) the atmospheric methane concentration has increased by about 160%, with the increase being overwhelmingly caused by human activity.[11] Since 1750 methane has contributed 3% of GHG emissions in terms of mass[12] but is responsible for approximately 23% of radiative or climate forcing.[13][14][15] In 2019, global methane concentrations rose from 722 parts per billion (ppb) in pre-industrial times to 1866 ppb.[16] This is an increase by a factor of 2.6 and the highest value in at least 800,000 years.[17]: 4 [18][19]
Methane increases the amount of ozone O3 in the troposphere (4 miles (6.4 km) to 12 miles (19 km) from the Earth's surface) and also in the stratosphere (from the troposphere to 31 miles (50 km) above the Earth's surface).[20] Both water vapour and ozone are GHGs, which in turn adds to climate warming.[5]: 2
Role in climate change

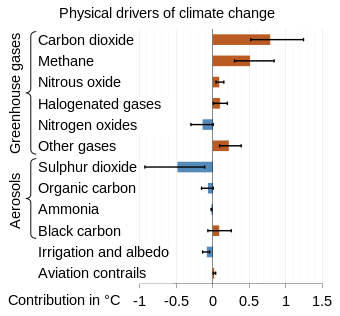
Methane in the Earth's atmosphere is a powerful greenhouse gas with a global warming potential (GWP) 84 times greater than CO2 in a 20-year time frame.[22][23]
Radiative or climate forcing is the scientific concept used to measure the human impact on the environment in watts / meter².[24] It refers to the "difference between solar irradiance absorbed by the Earth and energy radiated back to space"[25] The direct radiative greenhouse gas forcing effect of methane relative to 1750 has been estimated at 0.5 W/m2 (watts per meter²) in the 2007 IPCC "Climate Change Synthesis Report 2007".[26]: 38
In their May 21, 2021 173-page "Global Methane Assessment", the UNEP and CCAP said that their "understanding of methane's effect on radiative forcing" improved with research by teams led by M. Etminan in 2016,[13] and William Collins in 2018,[5] which resulted in an "upward revision" since the 2014 IPCC Fifth Assessment Report (AR5). The "improved understanding" says that prior estimates of the "overall societal impact of methane emissions" were likely underestimated.[27]: 18
Etminan et al. published their new calculations for methane's radiative forcing (RF) in a 2016 Geophysical Research Letters journal article which incorporated the shortwave bands of CH4 in measuring forcing, not used in previous, simpler IPCC methods. Their new RF calculations which significantly revised those cited in earlier, successive IPCC reports for well mixed greenhouse gases (WMGHG) forcings by including the shortwave forcing component due to CH4, resulted in estimates that were approximately 20-25% higher.[13] Collins et al. said that CH4 mitigation that reduces atmospheric methane by the end of the century, could "make a substantial difference to the feasibility of achieving the Paris climate targets," and would provide us with more "allowable carbon emissions to 2100".[5]
Methane is a strong GHG with a global warming potential 84 times greater than CO2 in a 20-year time frame. Methane is not as persistent a gas and tails off to about 28 times greater than CO2 for a 100-year time frame.[9]
In addition to the direct heating effect and the normal feedbacks, the methane breaks down to carbon dioxide and water. This water is often above the tropopause where little water usually reaches. Ramanathan (1988)[28] notes that both water and ice clouds, when formed at cold lower stratospheric temperatures, are extremely efficient in enhancing the atmospheric greenhouse effect. He also notes that there is a distinct possibility that large increases in future methane may lead to a surface warming that increases nonlinearly with the methane concentration.
Mitigation efforts to reduce short-lived climate pollutants, like methane and black carbon would help combat "near-term climate change" and would support Sustainable Development Goals.[29]
Sources
thumb|upright=1.8|Main sources of global methane emissions (2008-2017) according to the [[Global Carbon Project[30]|right]] Any process that results in the production of methane and its release into the atmosphere can be considered a "source". The known sources of methane are predominantly located near the Earth's surface.[12] Two main processes that are responsible for methane production include microorganisms anaerobically converting organic compounds into methane (methanogenesis), which are widespread in aquatic ecosystems, and ruminant animals. Other natural sources include melting permafrost, wetlands, plants, and methane clathrates.[citation needed]
Measurement techniques
Methane was typically measured using gas chromatography. Gas chromatography is a type of chromatography used for separating or analyzing chemical compounds. It is less expensive in general, compared to more advanced methods, but it is more time and labor-intensive.[citation needed]
Spectroscopic methods were the preferred method for atmospheric gas measurements due to its sensitivity and precision. Also, spectroscopic methods are the only way of remotely sensing the atmospheric gases. Infrared spectroscopy covers a large spectrum of techniques, one of which detects gases based on absorption spectroscopy. There are various methods for spectroscopic methods, including Differential optical absorption spectroscopy, Laser-induced fluorescence, and Fourier Transform Infrared.[citation needed]
In 2011, cavity ring-down spectroscopy was the most widely used IR absorption technique of detecting methane. It is a form of laser absorption spectroscopy which determines the mole fraction to the order of parts per trillion.
Global monitoring
thumb|upright=1.8|Methane concentration at NOAA's Mauna Loa observatory through July 2021: A record-high of 1912 ppb was reached in December 2020.[32]CH4 has been measured directly in the environment since the 1970s.[33][11] The Earth's atmospheric methane concentration has increased 160% since preindustrial levels in the mid-18th century.[11]
Long term atmospheric measurements of methane by NOAA show that the build up of methane nearly tripled since pre-industrial times since 1750.[34] In 1991 and 1998 there was a sudden growth rate of methane representing a doubling of growth rates in previous years.[34] The June 15, 1991 eruption of Mount Pinatubo, measuring VEI-6—was the second-largest terrestrial eruption of the 20th century.[35] In 2007 it was reported that unprecedented warm temperatures in 1998—the warmest year since surface records were recorded—could have induced elevated methane emissions, along with an increase in wetland and rice field emissions and the amount of biomass burning.[36]
Data from 2007 suggested methane concentrations were beginning to rise again.[37] This was confirmed in 2010 when a study showed methane levels were on the rise for the 3 years 2007 to 2009. After a decade of near-zero growth in methane levels, "globally averaged atmospheric methane increased by [approximately] 7 nmol/mol per year during 2007 and 2008. During the first half of 2009, globally averaged atmospheric CH4 was [approximately] 7 nmol/mol greater than it was in 2008, suggesting that the increase will continue in 2009."[38] From 2015 to 2019 sharp rises in levels of atmospheric methane have been recorded.[39]
In 2010, methane levels in the Arctic were measured at 1850 nmol/mol which is over twice as high as at any time in the last 400,000 years.[citation needed] According to the IPCC AR5, since 2011 concentrations continued to increase. After 2014, the increase accelerated and by 2017, it reached 1,850 (parts per billion) ppb.[40] The annual average for methane (CH4) was 1866 ppb in 2019 and scientists reported with "very high confidence" that concentrations of CH4 were higher than at any time in at least 800,000 years.[14] The largest annual increase occurred in 2021 with current concentrations reaching a record 260% of pre-industrial—with the overwhelming percentage caused by human activity.[11]
In 2013, IPCC scientists said with "very high confidence", that concentrations of atmospheric methane CH4 "exceeded the pre-industrial levels by about 150% which represented "levels unprecedented in at least the last 800,000 years."[14][41] The globally averaged concentration of methane in Earth's atmosphere increased by about 150% from 722 ± 25 ppb in 1750 to 1803.1 ± 0.6 ppb in 2011.[42][43] As of 2016, methane contributed radiative forcing of 0.62 ± 14% Wm−2,[13] or about 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases.[9] The atmospheric methane concentration has continued to increase since 2011 to an average global concentration of 1911.8 ± 0.6 ppb as of 2022.[16] The May 2021 peak was 1891.6 ppb, while the April 2022 peak was 1909.4 ppb, a 0.9% increase.[43]

The Global Carbon Project consortium produces the Global Methane Budget. Working with over fifty international research institutions and 100 stations globally, it updates the methane budget every few years.[44]
In 2013, the balance between sources and sinks of methane was not yet fully understood. Scientists were unable to explain why the atmospheric concentration of methane had temporarily ceased to increase.[45]
The focus on the role of methane in anthropogenic climate change has become more relevant since the mid-2010s.[46]
Natural sinks or removal of atmospheric methane
The amount of methane in the atmosphere is the result of a balance between the production of methane on the Earth's surface—its source—and the destruction or removal of methane, mainly in the atmosphere—its sink— in an atmospheric chemical process.[47]
Another major natural sink is through oxidation by methanotrophic or methane-consuming bacteria in Earth's soils.
thumb|upright=1.8|left|NASA computer models from 2005, calculated based on information available at that time, show the amount of methane (parts per million by volume) at the surface (top) and in the stratosphere (bottom)[48]These 2005 NASA computer model simulations—calculated based on data available at that time—illustrate how methane is destroyed as it rises. As air rises in the tropics, methane is carried upwards through the troposphere—the lowest portion of Earth's atmosphere which is 4 miles (6.4 km) to 12 miles (19 km) from the Earth's surface, into the lower stratosphere—the ozone layer—and then the upper portion of the stratosphere.[47]
This atmospheric chemical process is the most effective methane sink, as it removes 90% of atmospheric methane.[45] This global destruction of atmospheric methane mainly occurs in the troposphere.[45]
Methane molecules react with hydroxyl radicals (OH)—the "major chemical scavenger in the troposphere" that "controls the atmospheric lifetime of most gases in the troposphere".[49] Through this CH4 oxidation process, atmospheric methane is destroyed and water vapor and carbon dioxide are produced.
While this decreases the concentration of methane in the atmosphere, it also increases radiative forcing because both water vapor and carbon dioxide are more powerful GHGs factors in terms of affecting the warming of Earth.
This additional water vapor in the stratosphere caused by CH4 oxidation, adds approximately 15% to methane's radiative forcing effect.[50][6]
By the 1980s, the global warming problem had been transformed by the inclusion of methane and other non-CO2 trace-gases—CFCs, N2O, and O3— on global warming, instead of focusing primarily on carbon dioxide.[51][52] Both water and ice clouds, when formed at cold lower stratospheric temperatures, have a significant impact by increasing the atmospheric greenhouse effect. Large increases in future methane could lead to a surface warming that increases nonlinearly with the methane concentration.[51][52]
Methane also affects the degradation of the ozone layer—the lowest layer of the stratosphere from about 15 to 35 kilometers (9 to 22 mi) above Earth, just above the troposphere.[53] NASA researchers in 2001, had said that this process was enhanced by global warming, because warmer air holds more water vapor than colder air, so the amount of water vapor in the atmosphere increases as it is warmed by the greenhouse effect. Their climate models based on data available at that time, had indicated that carbon dioxide and methane enhanced the transport of water into the stratosphere.[54]
Atmospheric methane could last about 120 years in the stratosphere until it is eventually destroyed through the hydroxyl radicals oxidation process.[55]
Mean lifespan
As of 2001, the mean lifespan of methane in the atmosphere was estimated at 9.6 years. However, increasing emissions of methane over time reduced the concentration of the hydroxyl radical in the atmosphere.[56] With less OH˚ to react with, the lifespan of methane could also increase, resulting in greater concentrations of atmospheric methane.[57]
By 2013, methane's mean lifetime in the atmosphere was estimated to be twelve years.[29][58]
The reaction of methane and chlorine atoms acts as a primary sink of Cl atoms and is a primary source of hydrochloric acid (HCl) in the stratosphere.[59]
CH4 + Cl → CH3 + HCl
The HCl produced in this reaction leads to catalytic ozone destruction in the stratosphere.[55]
Methanotrophs in soils
Soils act as a major sink for atmospheric methane through the methanotrophic bacteria that reside within them. This occurs with two different types of bacteria. "High capacity-low affinity" methanotrophic bacteria grow in areas of high methane concentration, such as waterlogged soils in wetlands and other moist environments. And in areas of low methane concentration, "low capacity-high affinity" methanotrophic bacteria make use of the methane in the atmosphere to grow, rather than relying on methane in their immediate environment.[60]
Forest soils act as good sinks for atmospheric methane because soils are optimally moist for methanotroph activity, and the movement of gases between soil and atmosphere (soil diffusivity) is high.[60] With a lower water table, any methane in the soil has to make it past the methanotrophic bacteria before it can reach the atmosphere.
Wetland soils, however, are often sources of atmospheric methane rather than sinks because the water table is much higher, and the methane can be diffused fairly easily into the air without having to compete with the soil's methanotrophs.
Methanotrophic bacteria in soils – Methanotrophic bacteria that reside within soil use methane as a source of carbon in methane oxidation.[60] Methane oxidation allows methanotrophic bacteria to use methane as a source of energy, reacting methane with oxygen and as a result producing carbon dioxide and water.
- CH4 + 2O2 → CO2 + 2H2O
Removal technologies
Atmospheric Methane Removal is a category of potential approaches being researched to accelerate the breakdown of methane once in the atmosphere to mitigate some of the impacts of climate change.[61]
Different methods to remove methane from the atmosphere include thermal-catalytic oxidation, photocatalytic oxidation, biological methanotrophic methane removal, concentration with zeolites or other porous solids, and separation by membranes.[62]
Methane concentrations in the geologic past
left |upright=1.8|thumb|Compilation of paleoclimatology data of methaneFrom 1996 to 2004, researchers in the European Project for Ice Coring in Antarctica (EPICA) project were able to drill and analyze gases trapped in the ice cores in Antarctica to reconstruct GHG concentrations in the atmosphere over the past 800,000 years".[63] They found that prior to approximately 900,000 years ago, the cycle of ice ages followed by relatively short warm periods lasted about 40,000 years, but by 800,000 years ago the time interval changed dramatically to cycles that lasted 100,000 years.[63] There were low values of GHG in ice ages, and high values during the warm periods.[63]
This 2016 EPA illustration above is a compilation of paleoclimatology showing methane concentrations over time based on analysis of gas bubbles from[64] EPICA Dome C, Antarctica—approximately 797,446 BCE to 1937 CE,[65] Law Dome, Antarctica—approximately 1008 CE to 1980 CE[66] Cape Grim, Australia—1985 CE to 2015 CE[67] Mauna Loa, Hawaii—1984 CE to 2015 CE[68] and Shetland Islands, Scotland: 1993 CE to 2001 CE[69]

The massive and rapid release of large volumes of methane gas from such sediments into the atmosphere has been suggested as a possible cause for rapid global warming events in the Earth's distant past, such as the Paleocene–Eocene Thermal Maximum,[71] and the Great Dying.[72]
In 2001, NASA's Goddard Institute for Space Studies and Columbia University's Center for Climate Systems Research scientists confirmed that other greenhouse gases apart from carbon dioxide were important factors in climate change in research presented at the annual meeting of the American Geophysical Union (AGU).[73] They offered a theory on the 100,000-year long Paleocene–Eocene Thermal Maximum that occurred approximately 55 million years ago. They posited that there was a vast release of methane that had previously been kept stable through "cold temperatures and high pressure...beneath the ocean floor". This methane release into the atmosphere resulted in the warming of the earth. A 2009 journal article in Science, confirmed NASA research that the contribution of methane to global warming had previously been underestimated.[74][75]
Early in the Earth's history carbon dioxide and methane likely produced a greenhouse effect. The carbon dioxide would have been produced by volcanoes and the methane by early microbes. During this time, Earth's earliest life appeared.[76] According to a 2003 article in the journal Geology, these first, ancient bacteria added to the methane concentration by converting hydrogen and carbon dioxide into methane and water. Oxygen did not become a major part of the atmosphere until photosynthetic organisms evolved later in Earth's history. With no oxygen, methane stayed in the atmosphere longer and at higher concentrations than it does today.[77]
References
- ↑ Dlugokencky, Ed (December 5, 2016). "Trends in Atmospheric Methane". Global Greenhouse Gas Reference Network. NOAA Earth System Research Laboratory. http://www.esrl.noaa.gov/gmd/ccgg/trends_ch4/.
- ↑ "Methane Tracker 2021". IEA (Paris). 2021. https://www.iea.org/reports/methane-tracker-2021. Retrieved March 21, 2023.License: CC BY 4.0
- ↑ "Methane in the atmosphere is surging, and that's got scientists worried". March 1, 2019. https://www.latimes.com/science/sciencenow/la-sci-sn-methane-atmosphere-accelerating-20190301-story.html.
- ↑ "IPCC AR4 SYR Appendix Glossary". 2007. http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_appendix.pdf.
- ↑ 5.0 5.1 5.2 5.3 5.4 Collins, William J.; Webber, Christopher P.; Cox, Peter M.; Huntingford, Chris; Lowe, Jason; Sitch, Stephen; Chadburn, Sarah E.; Comyn-Platt, Edward et al. (April 20, 2018). "Increased importance of methane reduction for a 1.5 degree target". Environmental Research Letters 13 (5): 054003. doi:10.1088/1748-9326/aab89c. ISSN 1748-9326. Bibcode: 2018ERL....13e4003C. https://iopscience.iop.org/article/10.1088/1748-9326/aab89c/pdf. Retrieved March 19, 2023.
- ↑ 6.0 6.1 Noël, Stefan et al. (2017). "Water Vapour and Methane Coupling in the Stratosphere observed with SCIAMACHY Solar Occultation Measurements". Atmospheric Chemistry and Physics (18): 4463–4476. doi:10.5194/acp-18-4463-2018. https://acp.copernicus.org/preprints/acp-2017-893/acp-2017-893.pdf. Retrieved 22 August 2021.
- ↑ Myhre, Gunnar (9 January 2007). "Radiative forcing due to stratospheric water vapour from CH4 oxidation". Geophysical Research Letters 34 (1). doi:10.1029/2006GL027472. Bibcode: 2007GeoRL..34.1807M.
- ↑ "Methane: The other important greenhouse gas". Environmental Defence Fund. https://www.edf.org/climate/methane-other-important-greenhouse-gas.
- ↑ 9.0 9.1 9.2 Myhre, Gunnar et al. (2013). Stocker, T.F.; Qin, D.; Plattner, G.-K. et al.. eds. Anthropogenic and Natural Radiative Forcing. Cambridge, United Kingdom and New York, USA: Cambridge University Press. http://www.climatechange2013.org/images/report/WG1AR5_Chapter08_FINAL.pdf. Retrieved 2016-12-22. See Table 8.7.
- ↑ Drew T. Shindell; Greg Faluvegi; Dorothy M. Koch; Gavin A. Schmidt; Nadine Unger; Susanne E. Bauer (2009). "Improved attribution of climate forcing to emissions". Science 326 (5953): 716–718. doi:10.1126/science.1174760. PMID 19900930. Bibcode: 2009Sci...326..716S. https://zenodo.org/record/1230902.
- ↑ 11.0 11.1 11.2 11.3 "Global Methane Assessment". United Nations Environment Programme and Climate and Clean Air Coalition (Nairobi): 12. 2022. https://wedocs.unep.org/bitstream/handle/20.500.11822/41108/methane_2030_SPM.pdf. Retrieved March 15, 2023.
- ↑ 12.0 12.1 Saunois, M. et al. (December 12, 2016). "The Global Methane Budget 2000–2012" (in en). Earth System Science Data 8 (2): 697–751. doi:10.5194/essd-8-697-2016. ISSN 1866-3508. Bibcode: 2016ESSD....8..697S. https://essd.copernicus.org/articles/8/697/2016/essd-8-697-2016.html. Retrieved 28 August 2020.
- ↑ 13.0 13.1 13.2 13.3 Etminan, M.; Myhre, G.; Highwood, E. J.; Shine, K. P. (December 27, 2016). "Radiative forcing of carbon dioxide, methane, and nitrous oxide: A significant revision of the methane radiative forcing" (in en). Geophysical Research Letters 43 (24): 12,614–12,623. doi:10.1002/2016gl071930. ISSN 0094-8276. Bibcode: 2016GeoRL..4312614E.
- ↑ 14.0 14.1 14.2 "Climate Change 2021. The Physical Science Basis. Summary for Policymakers. Working Group I contribution to the WGI Sixth Assessment Report of the Intergovernmental Panel on Climate Change". The Intergovernmental Panel on Climate Change. https://www.ipcc.ch/assessment-report/ar6/.
- ↑ Ritchie, Hannah; Roser, Max; Rosado, Pablo (May 11, 2020). "CO₂ and Greenhouse Gas Emissions". Our World in Data. https://ourworldindata.org/greenhouse-gas-emissions. Retrieved March 19, 2023.
- ↑ 16.0 16.1 Laboratory, US Department of Commerce, NOAA, Earth System Research (2023-07-05). "Globally averaged marine surface annual mean data" (in EN-US). ESRL Global Monitoring Division – Global Greenhouse Gas Reference Network. https://gml.noaa.gov/webdata/ccgg/trends/ch4/ch4_annmean_gl.txt.
- ↑ Synthesis Report of the IPCC Sixth Assessment Report (AR6) (Report). Summary for Policy Makers. March 19, 2023. pp. 36. https://report.ipcc.ch/ar6syr/pdf/IPCC_AR6_SYR_SPM.pdf. Retrieved March 20, 2023.
- ↑ IPCC AR5 WG1 (2013). "Climate Change 2013: The Physical Science Basis – Summary for Policymakers". Cambridge University Press. http://www.climatechange2013.org/images/report/WG1AR5_SPM_FINAL.pdf.
- ↑ Mann, Michael E., ed. "Radiative forcing". Encyclopædia Britannica. https://www.britannica.com/science/radiative-forcing. Retrieved March 19, 2023.
- ↑ Wuebbles, Donald J.; Tamaresis, John S. (1993). "The Role of Methane in the Global Environment". in Khalil, M. A. K.. Atmospheric Methane: Sources, Sinks, and Role in Global Change. NATO ASI Series. Berlin, Heidelberg: Springer. pp. 469–513. doi:10.1007/978-3-642-84605-2_20. ISBN 978-3-642-84605-2.
- ↑ "The NOAA Annual Greenhouse Gas Index (AGGI)". National Oceanographic and Atmospheric Administration (NOAA). Spring 2023. https://gml.noaa.gov/aggi/aggi.html.
- ↑ Stocker. "Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change". Cambridge University Press. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_all_final.pdf.
- ↑ Stocker, Thomas, ed. Climate change 2013 : the physical science basis : Working Group I contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York. p. 166. ISBN 978-1-10741-532-4. OCLC 881236891. https://books.google.com/books?id=o4gaBQAAQBAJ.
- ↑ Drew, Shindell (2013). "Climate Change 2013: The Physical Science Basis – Working Group 1 contribution to the IPCC Fifth Assessment Report: Radiative Forcing in the AR5". Rutgers University. http://climate.envsci.rutgers.edu/climdyn2013/IPCC/IPCC_WGI12-RadiativeForcing.pdf.
- ↑ Rebecca, Lindsey (14 January 2009). "Climate and Earth's Energy Budget: Feature Articles". https://earthobservatory.nasa.gov/Features/EnergyBalance/page7.php.
- ↑ "Climate Change Synthesis Report 2007". United Nations Environment Programme. pp. 112. https://www.ipcc.ch/site/assets/uploads/2018/02/ar4_syr_full_report.pdf.
- ↑ Shindell, Drew, ed (May 6, 2021). "Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions". United Nations Environment Programme: 173. ISBN 978-92-807-3854-4. https://www.unep.org/resources/report/global-methane-assessment-benefits-and-costs-mitigating-methane-emissions.
- ↑ "Ramanathan". Trace-Gas Greenhouse Effect and Global Warming: Underlying Principles and Outstanding Issues. Ambio-Royal Swedish Academy of sciences. http://www.ipcc.ch/publications_and_data/ar4/syr/en/figure-2-4.html.
- ↑ 29.0 29.1 "Primer on Short-Lived Climate Pollutants". Climate & Clean Air Coalition. https://www.ccacoalition.org/en/resources/primer-short-lived-climate-pollutants.
- ↑ Saunois, M. et al. (July 15, 2020). "The Global Methane Budget 2000–2017" (in en). Earth System Science Data (ESSD) 12 (3): 1561–1623. doi:10.5194/essd-12-1561-2020. ISSN 1866-3508. Bibcode: 2020ESSD...12.1561S. https://essd.copernicus.org/articles/12/1561/2020/. Retrieved 28 August 2020.
- ↑ Nakaema, Walter M.; Hao, Zuo-Qiang; Rohwetter, Philipp; Wöste, Ludger; Stelmaszczyk, Kamil (2011-01-27). "PCF-Based Cavity Enhanced Spectroscopic Sensors for Simultaneous Multicomponent Trace Gas Analysis" (in en). Sensors 11 (2): 1620–1640. doi:10.3390/s110201620. ISSN 1424-8220. PMID 22319372. Bibcode: 2011Senso..11.1620N.
- ↑ gases/ch4/in-situ/surface/mlo/ch4 mlo surface-insitu 1 ccgg MonthlyData.txt "ESRL/GMD FTP Data Finder". ftp://aftp.cmdl.noaa.gov/data/trace gases/ch4/in-situ/surface/mlo/ch4 mlo surface-insitu 1 ccgg MonthlyData.txt.
- ↑ Fox-Kemper, B.; Hewitt, H.T.; Xiao, C.; Aðalgeirsdóttir, G.; Drijfhout, S.S.; Edwards, T.L.; Golledge, N.R.; Hemer, M. et al. (2021). Masson-Delmotte, V.; Zhai, P.; Pirani, A. et al.. eds. "Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks". Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK and New York, NY, USA). doi:10.1017/9781009157896.011. https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Full_Report.pdf.
- ↑ 34.0 34.1 "Ch.2 Changes in Atmospheric Constituents and in Radiative Forcing". Climate Change 2007 IPCC Fourth Assessment Report. IPPC. http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-3-2.html.
- ↑ "Scientists pinpoint cause of slowing methane emissions". National Oceanic & Atmospheric Administration news Online. 2006-09-28. http://www.noaanews.noaa.gov/stories2006/s2709.htm.
- ↑ Denman, K.L.. "7. Couplings Between Changes in the Climate System and Biogeochemistry.". IPCC AR4 WG1 2007. http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch7.html.
- ↑ "Annual Greenhouse Gas Index (AGGI) Indicates Sharp Rise in Carbon Dioxide and Methane in 2007". National Oceanic & Atmospheric Administration – Earth System Research Laboratory. 2008-04-23. http://www.esrl.noaa.gov/news/2008/aggi.html.
- ↑ Heidi Blake (February 22, 2010). "Climate change could be accelerated by 'methane time bomb'". The Telegraph. https://www.telegraph.co.uk/earth/environment/climatechange/7289698/Climate-change-could-be-accelerated-by-methane-time-bomb.html.
- ↑ McKie, Robin (2019-02-17). "Sharp rise in methane levels threatens world climate targets" (in en-GB). The Observer. ISSN 0029-7712. https://www.theguardian.com/environment/2019/feb/17/methane-levels-sharp-rise-threaten-paris-climate-agreement.
- ↑ Nisbet, E. G.; Manning, M. R.; Dlugokencky, E. J.; Fisher, R. E.; Lowry, D.; Michel, S. E.; Myhre, C. Lund; Platt, S. M. et al. (2019). "Very Strong Atmospheric Methane Growth in the 4 Years 2014–2017: Implications for the Paris Agreement". Global Biogeochemical Cycles 33 (3): 318–342. doi:10.1029/2018GB006009. ISSN 1944-9224. Bibcode: 2019GBioC..33..318N.
- ↑ IPCC (2013). Stocker, T. F.; Qin, D.; Plattner, G.-K. et al.. eds. Climate Change 2013: The Physical Science Basis (Report). Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. https://www.ipcc.ch/site/assets/uploads/2018/03/WG1AR5_SummaryVolume_FINAL.pdf.
- ↑ Stocker. "Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change". p. 182. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_all_final.pdf.
- ↑ 43.0 43.1 Laboratory, US Department of Commerce, NOAA, Earth System Research (2023-07-05). "Globally averaged marine surface monthly mean data" (in EN-US). ESRL Global Monitoring Division – Global Greenhouse Gas Reference Network. https://gml.noaa.gov/webdata/ccgg/trends/ch4/ch4_mm_gl.txt.
- ↑ Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Gregor, Luke; Hauck (2022-11-11). "Global Carbon Budget 2022". Earth System Science Data 14 (11): 4811–4900. doi:10.5194/essd-14-4811-2022. ISSN 1866-3516. Bibcode: 2022ESSD...14.4811F. https://essd.copernicus.org/articles/14/4811/2022/. Retrieved 2023-03-15.
- ↑ 45.0 45.1 45.2 Kirschke, Stefanie et al. (September 22, 2013). "Three decades of global methane sources and sinks". Nature Geoscience 6 (10): 813–823. doi:10.1038/ngeo1955. Bibcode: 2013NatGe...6..813K. https://zenodo.org/record/3425876.
- ↑ Saunois, M; Jackson, B.; Bousquet, P.; Poulter, B.; Canadell, J G (2016). "The growing role of methane in anthropogenic climate change". Environmental Research Letters 11 (120207): 120207. doi:10.1088/1748-9326/11/12/120207.
- ↑ 47.0 47.1 "GMAO Chemical Forecasts and GEOS–CHEM NRT Simulations for ICARTT (top) and Randy Kawa, NASA GSFC Atmospheric Chemistry and Dynamics Branch (lower)". http://earthobservatory.nasa.gov/Newsroom/NewImages/images.php3?img_id=16827.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedNASA 2005 - ↑ Levine, S.. "Chemistry of the Hydroxyl Radical (OH) in the Troposphere". Treatise on geochemistry. 5 (2 ed.). Oxford: Elsevier Science.
- ↑ Myhre, Gunnar (January 9, 2007). "Radiative forcing due to stratospheric water vapour from CH4 oxidation". Geophysical Research Letters 34 (1). doi:10.1029/2006GL027472. Bibcode: 2007GeoRL..34.1807M.
- ↑ 51.0 51.1 Ramanathan, V. (1998). "Trace-Gas Greenhouse Effect and Global Warming: Underlying Principles and Outstanding Issues Volvo Environmental Prize Lecture-1997". Ambio 27 (3): 187–197. ISSN 0044-7447. https://www.jstor.org/stable/4314715. Retrieved March 23, 2023.
- ↑ 52.0 52.1 "Ramanathan". Trace-Gas Greenhouse Effect and Global Warming: Underlying Principles and Outstanding Issues. Ambio-Royal Swedish Academy of Sciences. http://www.ipcc.ch/publications_and_data/ar4/syr/en/figure-2-4.html.
- ↑ "Ozone Basics". 2008-03-20. http://www.ozonelayer.noaa.gov/science/basics.htm.
- ↑ Shindell, Drew (2001). "Wetter Upper Atmosphere May Delay Global Ozone Recovery". NASA. http://www.giss.nasa.gov/research/news/20010417/.
- ↑ 55.0 55.1 Rohs, S.; Schiller, C.; Riese, M.; Engel, A.; Schmidt, U.; Wetter, T.; Levin, I.; Nakazawa, T. (July 2006). "Long-term changes of methane and hydrogen in the stratosphere in the period 1978–2003 and their impact on the abundance of stratospheric water vapor". Journal of Geophysical Research: Atmospheres 111 (D14): D14315. doi:10.1029/2005JD006877. D14315. Bibcode: 2006JGRD..11114315R. http://juser.fz-juelich.de/record/50145/files/2005JD006877.pdf.
- ↑ "Methane and Nitrous Oxide Emissions From Natural Sources". USA Environmental Protection Agency Office of Atmospheric Programs. April 2010. http://www.epa.gov/methane/pdfs/Methane-and-Nitrous-Oxide-Emissions-From-Natural-Sources.pdf.
- ↑ Holmes, C. D. et al. (January 2013). "Future methane, hydroxyl, and their uncertainties: key climate and emission parameters for future predictions". Atmospheric Chemistry and Physics 13 (1): 285–302. doi:10.5194/acp-13-285-2013. Bibcode: 2013ACP....13..285H. https://escholarship.org/content/qt5rc7k3s3/qt5rc7k3s3.pdf?t=n4jknv. See Table 2.
- ↑ How long do greenhouse gases stay in the air?
- ↑ Warneck, Peter (2000) (in en). Chemistry of the Natural Atmosphere. Academic Press. ISBN 9780127356327. https://books.google.com/books?id=dm4RAQAAIAAJ.
- ↑ 60.0 60.1 60.2 Reay, Dave. "Methane Sinks − Soils". Greenhouse Gas Online. http://www.ghgonline.org/methanesinksoil.htm.
- ↑ Jackson, Robert (2021). "Atmospheric methane removal: a research agenda". Philosophical Transactions A 379 (20200454). doi:10.1098/rsta.2020.0454. PMID 34565221. Bibcode: 2021RSPTA.37900454J.
- ↑ Nisbet-Jones, Peter B. R.; Fernandez, Julianne M.; Fisher, Rebecca E.; France, James L.; Lowry, David; Waltham, David A.; Woolley Maisch, Ceres A.; Nisbet, Euan G. (24 January 2022). "Is the destruction or removal of atmospheric methane a worthwhile option?". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 380 (2215). doi:10.1098/rsta.2021.0108. PMID 34865528. Bibcode: 2022RSPTA.38010108N.
- ↑ 63.0 63.1 63.2 "The oldest ice on Earth may be able to solve the puzzle of the planet's climate history". University of Bern. April 2019. https://phys.org/news/2019-04-oldest-ice-earth-puzzle-planet.html.
- ↑ Climate Change Indicators in the United States: Atmospheric Concentrations of Greenhouse Gases (Report). August 2016. https://www.epa.gov/sites/default/files/2016-08/documents/print_ghg-concentrations-2016.pdf. Retrieved March 20, 2023.
- ↑ Loulergue, Laetitia; Schilt, Adrian; Spahni, Renato; Masson-Delmotte, Valérie; Blunier, Thomas; Lemieux, Bénédicte; Barnola, Jean-Marc; Raynaud, Dominique et al. (May 15, 2008). "Orbital and millennial-scale features of atmospheric CH4 over the past 800,000 years". Nature 453 (7193): 383–386. doi:10.1038/nature06950. ISSN 1476-4687. PMID 18480822. Bibcode: 2008Natur.453..383L. https://www.ncdc.noaa.gov/paleo/pubs/loulergue2008/loulergue2008.html. Retrieved March 20, 2023.
- ↑ Etheridge, D.; Steele, L.; Francey, R.; Langenfelds, R. (2002). Historic CH4 Records from Antarctic and Greenland Ice Cores, Antarctic Firn Data, and Archived Air Samples from Cape Grim, Tasmania (Report). Trends: A compendium of data on global change. Oak Ridge, TN: Environmental System Science Data Infrastructure for a Virtual Ecosystem; Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge National Laboratory (ORNL). doi:10.3334/CDIAC/ATG.030. https://www.osti.gov/servlets/purl/1394397/. Retrieved March 21, 2023.
- ↑ "Monthly mean CH4 concentrations for Cape Grim, Australia". National Oceanic and Atmospheric Administration. 2016.
- ↑ "Monthly mean CH4 concentrations for Mauna Loa, Hawaii". National Oceanic and Atmospheric Administration. 2016.
- ↑ Steele, L.P.; Krummel, P.B.; Langenfelds, R.L. (October 2002). Atmospheric methane record from Shetland Islands, Scotland. Trends: A compendium of data on global change. Oak Ridge, TN. https://cdiac.ess-dive.lbl.gov/trends/atm_meth/csiro/csiro-shetlandch4.html. Retrieved March 20, 2023.
- ↑ "Methane | Reg Morrison" (in en-US). http://regmorrison.edublogs.org/2011/09/26/methane/.
- ↑ Bowen, Gabriel J. et al. (December 15, 2014). "Two massive, rapid releases of carbon during the onset of the Palaeocene–Eocene thermal maximum". Nature Geoscience 8 (1): 44–47. doi:10.1038/ngeo2316. Bibcode: 2015NatGe...8...44B.
- ↑ Benton, Michael J.; Twitchett, Richard J. (July 2003). "How to kill (almost) all life: the end-Permian extinction event". Trends in Ecology & Evolution 18 (7): 358–365. doi:10.1016/S0169-5347(03)00093-4.
- ↑ "Methane Explosion Warmed The Prehistoric Earth, Possible Again". NASA/Goddard Space Flight Center, EOS Project Science Office (Press release). December 12, 2001. Retrieved March 22, 2023 – via ScienceDaily.
- ↑ Shindell, 2 Greg; Faluvegi, G.; Koch, Dorothy M.; Schmidt, Gavin A.; Unger, Nadine; Bauer, Susanne E. (October 30, 2009). "Improved Attribution of Climate Forcing to Emissions". Science 326 (5953): 716–718. doi:10.1126/science.1174760. PMID 19900930. Bibcode: 2009Sci...326..716S. https://zenodo.org/record/1230902.
- ↑ Vergano, Dan (2009-10-29). "Methane's role in global warming underestimated". USA Today. https://www.usatoday.com/tech/science/environment/2009-10-29-methane-global-warming_N.htm.
- ↑ Gale, Joseph (2009). Astrobiology of Earth: the emergence, evolution, and future of life on a planet in turmoil. Oxford: Oxford University Press. ISBN 978-0-19-920580-6. https://books.google.com/books?id=64zn0nxDVUYC.
- ↑ Pavlov, Alexander A. et al. (January 2003). "Methane-rich Proterozoic atmosphere?". Geology 31 (1): 87–90. doi:10.1130/0091-7613(2003)031<0087:MRPA>2.0.CO;2. Bibcode: 2003Geo....31...87P.
 |
