Physics:Iodine-131
 | |
| General | |
|---|---|
| Symbol | 131I |
| Names | iodine-131, I-131, radioiodine |
| Protons | 53 |
| Neutrons | 78 |
| Nuclide data | |
| Half-life | 8.0249 d[1] |
| Decay products | 131Xe |
| Isotope mass | 130.906126Template:AME2020 II u |
| Spin | 7/2+[1] |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β− + γ | 0.971[2] |
| Isotopes of Chemistry:iodine Complete table of nuclides | |
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley.[3] It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (see fission product yield).
Due to its beta decay, iodine-131 causes mutation and death in cells that it penetrates, and other cells up to several millimeters away. For this reason, high doses of the isotope are sometimes less dangerous than low doses, since they tend to kill thyroid tissues that would otherwise become cancerous as a result of the radiation. For example, children treated with moderate dose of 131I for thyroid adenomas had a detectable increase in thyroid cancer, but children treated with a much higher dose did not.[4] Likewise, most studies of very-high-dose 131I for treatment of Graves' disease have failed to find any increase in thyroid cancer, even though there is linear increase in thyroid cancer risk with 131I absorption at moderate doses.[5] Thus, iodine-131 is increasingly less employed in small doses in medical use (especially in children), but increasingly is used only in large and maximal treatment doses, as a way of killing targeted tissues (i.e. therapeutic use).
Iodine-131 can be "seen" by nuclear medicine imaging techniques (e.g., gamma cameras) whenever it is given for therapeutic use, since it is a strong emitter of gamma radiation. However, since the beta radiation causes tissue damage without contributing to any ability to see or "image" the isotope, other less-damaging radioisotopes of iodine such as iodine-123 (see isotopes of iodine) are preferred in situations when only imaging is wanted. The isotope 131I is still occasionally used for purely diagnostic (i.e., imaging) work, due to its low expense compared to other iodine radioisotopes. No increase in thyoid cancer has been seen from the small medical imaging doses of 131I. The low-cost availability of 131I, in turn, is due to the relative ease of creating 131I by neutron bombardment of natural tellurium in a nuclear reactor, then separating 131I out by various simple methods (i.e., heating to drive off the volatile iodine). By contrast, other iodine radioisotopes are usually created by far more expensive techniques, starting with cyclotron radiation of capsules of pressurized xenon gas.[6]
Iodine-131 is also one of the most commonly used gamma-emitting radioactive industrial tracer. Radioactive tracer isotopes are injected with hydraulic fracturing fluid to determine the injection profile and location of fractures created by hydraulic fracturing.[7]
Much smaller incidental doses of iodine-131 than those used in medical therapeutic procedures, are concluded by some studies to be the major cause of increased thyroid cancers after exposure to nuclear fission products.[8][9] Other studies did not find a correlation.[10][11]
Production
Most 131I production is from neutron irradiation of a natural tellurium target in a nuclear reactor. Irradiation of natural tellurium produces almost entirely 131I as the only radionuclide with a half-life longer than hours (and shorter than millions of years), since lighter isotopes of tellurium become heavier stable isotopes, or else stable antimony or iodine. However, the heaviest naturally occurring tellurium nuclide, 130Te (34% of natural tellurium) absorbs a neutron to become tellurium-131, which beta decays with a half-life of 25 minutes to 131I.
A tellurium compound can be irradiated while bound as an oxide to an ion exchange column, with evolved 131I then eluted into an alkaline solution.[12] More commonly, powdered elemental tellurium is irradiated and then 131I separated from it by dry distillation of the iodine, which has a far higher vapor pressure. The element is then dissolved in a mildly alkaline solution in the standard manner, to produce 131I as iodide and hypoiodate (which is soon reduced to iodide).[13]
131I is a fission product with a yield of 2.878% from uranium-235,[14] and can be released in nuclear weapons tests and nuclear accidents. However, the short half-life means it is not present in significant quantities in cooled spent nuclear fuel, unlike iodine-129 whose half-life is nearly a billion times that of 131I.
It is discharged to the atmosphere in small quantities by some nuclear power plants.[15]
Radioactive decay

131I decays with a half-life of 8.0249 days[1] emitting beta particles and gamma rays. Most often (89%), 131I most often expends its 971 keV of decay energy by transforming to stable xenon-131 in two steps, with gamma decay following rapidly after beta decay:
The primary emissions of 131I decay are thus electrons with a maximal energy of 606 keV and gammas of 364 keV.[2] Beta decay also produces an antineutrino, which carries off variable amounts of the energy. The electrons, due to their high mean energy (190 keV, with a typical beta-decay spectrum) have a tissue penetration of 0.6 to 2 mm.[16]
Effects of exposure
Iodine in food is absorbed by the body and preferentially concentrated in the thyroid where it is needed for the functioning of that gland. When 131I is present in high levels in the environment from radioactive fallout, it can be absorbed through contaminated food, and will also accumulate in the thyroid. As it decays, it may cause damage to the thyroid. The primary risk from exposure to 131I is an increased risk of radiation-induced cancer in later life. Other risks include the possibility of non-cancerous growths and thyroiditis.[5]
The risk of thyroid cancer in later life appears to diminish with increasing age at time of exposure. Most risk estimates are based on studies in which radiation exposures occurred in children or teenagers. When adults are exposed, it has been difficult for epidemiologists to detect a statistically significant difference in the rates of thyroid disease above that of a similar but otherwise-unexposed group.[5][18]
The risk can be mitigated by taking iodine supplements, raising the total amount of iodine in the body and, therefore, reducing uptake and retention in the face and chest and lowering the relative proportion of radioactive iodine. However, such supplements were not consistently distributed to the population living nearest to the Chernobyl nuclear power plant after the disaster,[19] though they were widely distributed to children in Poland.
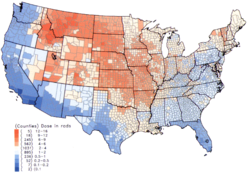
Within the US, the highest 131I fallout doses occurred during the 1950s and early 1960s to children having consumed fresh milk from sources contaminated as the result of above-ground testing of nuclear weapons.[8] The National Cancer Institute provides additional information on the health effects from exposure to 131I in fallout,[20] as well as individualized estimates, for those born before 1971, for each of the 3070 counties in the US. The calculations are taken from data collected regarding fallout from the nuclear weapons tests conducted at the Nevada Test Site.[21]
On 27 March 2011, the Massachusetts Department of Public Health reported that 131I was detected in very low concentrations in rainwater from samples collected in Massachusetts, and that this likely originated from the Fukushima power plant.[22] Farmers near the plant dumped raw milk, while testing in the United States found 0.8 pico-curies per liter of iodine-131 in a milk sample, but the radiation levels were 5,000 times lower than the FDA's "defined intervention level".
Treatment and prevention
A common treatment method for preventing iodine-131 exposure is by saturating the thyroid with regular, stable iodine-127, as an iodide or iodate salt.
Medical use
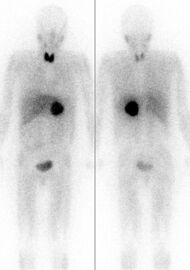
Iodine-131 is used for unsealed source radiotherapy in nuclear medicine to treat several conditions. It can also be detected by gamma cameras for diagnostic imaging, however it is rarely administered for diagnostic purposes only; imaging will normally be done following a therapeutic dose.[24] Use of the 131I as iodide salt exploits the mechanism of absorption of iodine by the normal cells of the thyroid gland.
Treatment of thyrotoxicosis
Major uses of 131I include the treatment of thyrotoxicosis (hyperthyroidism) due to Graves' disease, and sometimes hyperactive thyroid nodules (abnormally active thyroid tissue that is not malignant). The therapeutic use of radioiodine to treat hyperthyroidism from Graves' disease was first reported by Saul Hertz in 1941. The dose is typically administered orally (either as a liquid or capsule), in an outpatient setting, and is usually 400–600 megabecquerels (MBq).[25] Radioactive iodine (iodine-131) alone can potentially worsen thyrotoxicosis in the first few days after treatment. One side effect of treatment is an initial period of a few days of increased hyperthyroid symptoms. This occurs because when the radioactive iodine destroys the thyroid cells, they can release thyroid hormone into the blood stream. For this reason, sometimes patients are pre-treated with thyrostatic medications such as methimazole, and/or they are given symptomatic treatment such as propranolol. Radioactive iodine treatment is contraindicated in breast-feeding and pregnancy[26]
Treatment of thyroid cancer
Iodine-131, in higher doses than for thyrotoxicosis, is used for ablation of remnant thyroid tissue following a complete thyroidectomy to treat thyroid cancer.[27][25]
Administration of I-131 for ablation
Typical therapeutic doses of I-131 are between 2220 and 7400 megabecquerels (MBq).[28] Because of this high radioactivity and because the exposure of stomach tissue to beta radiation would be high near an undissolved capsule, I-131 is sometimes administered to human patients in a small amount of liquid. Administration of this liquid form is usually by straw which is used to slowly and carefully suck up the liquid from a shielded container.[29] For administration to animals (for example, cats with hyperthyroidism), for practical reasons the isotope must be administered by injection. European guidelines recommend administration of a capsule, due to "greater ease to the patient and the superior radiation protection for caregivers".[30]
Post-treatment isolation
Ablation doses are usually administered on an inpatient basis, and IAEA International Basic Safety Standards recommend that patients are not discharged until the activity falls below 1100 MBq.[31] ICRP advice states that "comforters and carers" of patients undergoing radionuclide therapy should be treated as members of the public for dose constraint purposes and any restrictions on the patient should be designed based on this principle.[32]
Patients receiving I-131 radioiodine treatment may be warned not to have sexual intercourse for one month (or shorter, depending on dose given), and women told not to become pregnant for six months afterwards. "This is because a theoretical risk to a developing fetus exists, even though the amount of radioactivity retained may be small and there is no medical proof of an actual risk from radioiodine treatment. Such a precaution would essentially eliminate direct fetal exposure to radioactivity and markedly reduce the possibility of conception with sperm that might theoretically have been damaged by exposure to radioiodine."[33] These guidelines vary from hospital to hospital and will depend on national legislation and guidance, as well as the dose of radiation given. Some also advise not to hug or hold children when the radiation is still high, and a one- or two- metre distance to others may be recommended.[34]
I-131 will be eliminated from the body over the next several weeks after it is given. The majority of I-131 will be eliminated from the human body in 3–5 days, through natural decay, and through excretion in sweat and urine. Smaller amounts will continue to be released over the next several weeks, as the body processes thyroid hormones created with the I-131. For this reason, it is advised to regularly clean toilets, sinks, bed sheets and clothing used by the person who received the treatment. Patients may also be advised to wear slippers or socks at all times, and avoid prolonged close contact with others. This minimizes accidental exposure by family members, especially children.[35] Use of a decontaminant specially made for radioactive iodine removal may be advised. The use of chlorine bleach solutions, or cleaners that contain chlorine bleach for cleanup, are not advised, since radioactive elemental iodine gas may be released.[36] Airborne I-131 may cause a greater risk of second-hand exposure, spreading contamination over a wide area. Patient is advised if possible to stay in a room with a bathroom connected to it to limit unintended exposure to family members.
Many airports have radiation detectors to detect the smuggling of radioactive materials. Patients should be warned that if they travel by air, they may trigger radiation detectors at airports up to 95 days after their treatment with 131I.[37]
Other therapeutic uses
The 131I isotope is also used as a radioactive label for certain radiopharmaceuticals that can be used for therapy, e.g. 131I-metaiodobenzylguanidine (131I-MIBG) for imaging and treating pheochromocytoma and neuroblastoma. In all of these therapeutic uses, 131I destroys tissue by short-range beta radiation. About 90% of its radiation damage to tissue is via beta radiation, and the rest occurs via its gamma radiation (at a longer distance from the radioisotope). It can be seen in diagnostic scans after its use as therapy, because 131I is also a gamma-emitter.
Diagnostic uses
Because of the carcinogenicity of its beta radiation in the thyroid in small doses, I-131 is rarely used primarily or solely for diagnosis (although in the past this was more common due to this isotope's relative ease of production and low expense). Instead the more purely gamma-emitting radioiodine iodine-123 is used in diagnostic testing (nuclear medicine scan of the thyroid). The longer half-lived iodine-125 is also occasionally used when a longer half-life radioiodine is needed for diagnosis, and in brachytherapy treatment (isotope confined in small seed-like metal capsules), where the low-energy gamma radiation without a beta component makes iodine-125 useful. The other radioisotopes of iodine are never used in brachytherapy.
The use of 131I as a medical isotope has been blamed for a routine shipment of biosolids being rejected from crossing the Canada—U.S. border.[38] Such material can enter the sewers directly from the medical facilities, or by being excreted by patients after a treatment.
Industrial radioactive tracer uses
Used for the first time in 1951 to localize leaks in a drinking water supply system of Munich, Germany, iodine-131 became one of the most commonly used gamma-emitting industrial radioactive tracers, with applications in isotope hydrology and leak detection.[39][40][41][42]
Since the late 1940s, radioactive tracers have been used by the oil industry. Tagged at the surface, water is then tracked downhole, using the appropriated gamma detector, to determine flows and detect underground leaks. I-131 has been the most widely used tagging isotope in an aqueous solution of sodium iodide.[43][44][45] It is used to characterize the hydraulic fracturing fluid to help determine the injection profile and location of fractures created by hydraulic fracturing.[46][47][48]
In popular culture
- The use of iodine-131 as a poison – used in small doses over a period of time to disrupt a person's ability to think and tell right from wrong – played a central role in the episode "The Case of the Melancholy Marksman" of the long-running CBS TV series Perry Mason (season 5, episode 24, first broadcast March 24, 1962).
See also
References
- ↑ 1.0 1.1 1.2 Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties". Chinese Physics C 45 (3). doi:10.1088/1674-1137/abddae. https://www-nds.iaea.org/amdc/ame2020/NUBASE2020.pdf.
- ↑ 2.0 2.1 National Nuclear Data Center. "NuDat 2.x database". Brookhaven National Laboratory. http://www.nndc.bnl.gov/nudat2/.
- ↑ "UW-L Brachy Course". wikifoundry. April 2008. http://uwlbrachycourse.wikifoundry.com/page/Iodine-131.
- ↑ Dobyns, B. M.; Sheline, G. E.; Workman, J. B.; Tompkins, E. A.; McConahey, W. M.; Becker, D. V. (June 1974). "Malignant and benign neoplasms of the thyroid in patients treated for hyperthyroidism: a report of the cooperative thyrotoxicosis therapy follow-up study". The Journal of Clinical Endocrinology and Metabolism 38 (6): 976–998. doi:10.1210/jcem-38-6-976. ISSN 0021-972X. PMID 4134013.
- ↑ 5.0 5.1 5.2 Rivkees, Scott A.; Sklar, Charles; Freemark, Michael (1998). "The Management of Graves' Disease in Children, with Special Emphasis on Radioiodine Treatment". Journal of Clinical Endocrinology & Metabolism 83 (11): 3767–76. doi:10.1210/jcem.83.11.5239. PMID 9814445.
- ↑ Rayyes, Al; Hamid, Abdul (2002) (in English) (pdf). Technical meeting of project counterparts on cyclotron production of I-123. IAEA. https://inis.iaea.org/search/search.aspx?orig_q=RN:33007368.
- ↑ Reis, John C. (1976). Environmental Control in Petroleum Engineering. Gulf Professional Publishers.
- ↑ 8.0 8.1 Simon, Steven L.; Bouville, André; Land, Charles E. (January–February 2006). "Fallout from Nuclear Weapons Tests and Cancer Risks". American Scientist 94 (1): 48–57. doi:10.1511/2006.1.48. "In 1997, NCI conducted a detailed evaluation of dose to the thyroid glands of U.S. residents from I-131 in fallout from tests in Nevada. (...) we evaluated the risks of thyroid cancer from that exposure and estimated that about 49,000 fallout-related cases might occur in the United States, almost all of them among persons who were under age 20 at some time during the period 1951–57, with 95-percent uncertainty limits of 11,300 and 212,000.".
- ↑ "National Cancer Institute calculator for thyroid cancer risk as a result of I-131 intake after nuclear testing before 1971 in Nevada". Ntsi131.nci.nih.gov. https://ntsi131.nci.nih.gov/.
- ↑ Guiraud-Vitaux, F.; Elbast, M.; Colas-Linhart, N.; Hindie, E. (February 2008). "Thyroid cancer after Chernobyl: is iodine 131 the only culprit ? Impact on clinical practice". Bulletin du Cancer 95 (2): 191–5. doi:10.1684/bdc.2008.0574. PMID 18304904.
- ↑ Hanford Thyroid Disease Study, Fred Hutchinson Cancer Research Center, 2002, https://www.cdc.gov/nceh/radiation/hanford/htdsweb/pdf/htdsreport.pdf, retrieved 17 June 2012, "no associations between Hanford's iodine-131 releases and thyroid disease were observed. [The findings] show that if there is an increased risk of thyroid disease from exposure to Hanford's iodine-131, it is probably too small to observe using the best epidemiologic methods available" Executive summary
- ↑ Chattopadhyay, Sankha; Saha Das, Sujata (2010). "Recovery of 131I from alkaline solution of n-irradiated tellurium target using a tiny Dowex-1 column". Applied Radiation and Isotopes 68 (10): 1967–9. doi:10.1016/j.apradiso.2010.04.033. PMID 20471848.
- ↑ "I-131 Fact Sheet". Nordion. August 2011. http://www.mds.nordion.com/documents/products/I-131_Solu_Can.pdf.
- ↑ "Nuclear Data for Safeguards, Table C-3, Cumulative Fission Yields". International Atomic Energy Agency. http://www-nds.iaea.org/sgnucdat/c3.htm. (thermal neutron fission)
- ↑ Effluent Releases from Nuclear Power Plants and Fuel-Cycle Facilities. National Academies Press (US). 2012-03-29. https://www.ncbi.nlm.nih.gov/books/NBK201991/.
- ↑ Skugor, Mario (2006). Thyroid Disorders. A Cleveland Clinic Guide. Cleveland Clinic Press. p. 82. ISBN 978-1-59624-021-6. https://archive.org/details/thyroiddisorders00mari/page/82.
- ↑ National Research Council (11 February 2003). Exposure of the American Population to Radioactive Fallout from Nuclear Weapons Tests: A Review of the CDC-NCI Draft Report on a Feasibility Study of the Health Consequences to the American Population from Nuclear Weapons Tests Conducted by the United States and Other Nations. Washington, D.C.: National Academies Press. doi:10.17226/10621. ISBN 978-0-309-08713-1. https://nap.nationalacademies.org/catalog/10621/exposure-of-the-american-population-to-radioactive-fallout-from-nuclear-weapons-tests. Retrieved 10 February 2025.
- ↑ Robbins, Jacob; Schneider, Arthur B. (2000). "Thyroid cancer following exposure to radioactive iodine". Reviews in Endocrine and Metabolic Disorders 1 (3): 197–203. doi:10.1023/A:1010031115233. ISSN 1389-9155. PMID 11705004.
- ↑ Frot, Jacques. "The Causes of the Chernobyl Event". Ecolo.org. http://www.ecolo.org/documents/documents_in_english/Causes.ChernobyJF.doc.
- ↑ "Radioactive I-131 from Fallout". National Cancer Institute. http://www.cancer.gov/i131.
- ↑ "Individual Dose and Risk Calculator for Nevada Test Site fallout". National Cancer Institute. 1 October 2007. http://ntsi131.nci.nih.gov/.
- ↑ "Low Concentrations Of Radiation Found In Mass. | WCVB Home – WCVB Home". Thebostonchannel.com. 2011-03-27. http://www.thebostonchannel.com/r-video/27338488/detail.html.
- ↑ Nakajo, M., Shapiro, B. Sisson, J.C., Swanson, D.P., and Beierwaltes, W.H. Salivary gland uptake of Meta-[I131]Iodobenzylguanidine. J Nucl Med 25:2–6, 1984
- ↑ Carpi, Angelo; Mechanick, Jeffrey I. (2016). Thyroid Cancer: From Emergent Biotechnologies to Clinical Practice Guidelines. CRC Press. p. 148. ISBN 9781439862223. https://books.google.com/books?id=nQwrGfR1rZoC&pg=PA148.
- ↑ 25.0 25.1 Stokkel, Marcel P. M.; Handkiewicz Junak, Daria; Lassmann, Michael; Dietlein, Markus; Luster, Markus (13 July 2010). "EANM procedure guidelines for therapy of benign thyroid disease". European Journal of Nuclear Medicine and Molecular Imaging 37 (11): 2218–2228. doi:10.1007/s00259-010-1536-8. PMID 20625722.
- ↑ Brunton, Laurence L. et al. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 12e. 2011. Chapter 39
- ↑ Silberstein, E. B.; Alavi, A.; Balon, H. R.; Clarke, S. E. M.; Divgi, C.; Gelfand, M. J.; Goldsmith, S. J.; Jadvar, H. et al. (11 July 2012). "The SNMMI Practice Guideline for Therapy of Thyroid Disease with 131I 3.0". Journal of Nuclear Medicine 53 (10): 1633–1651. doi:10.2967/jnumed.112.105148. PMID 22787108.
- ↑ Yama, Naoya; Sakata, Koh-ichi; Hyodoh, Hideki; Tamakawa, Mitsuharu; Hareyama, Masato (June 2012). "A retrospective study on the transition of radiation dose rate and iodine distribution in patients with I-131-treated well-differentiated thyroid cancer to improve bed control shorten isolation periods". Annals of Nuclear Medicine 26 (5): 390–396. doi:10.1007/s12149-012-0586-3. ISSN 1864-6433. PMID 22382609.
- ↑ Rao, V. P.; Sudhakar, P.; Swamy, V. K.; Pradeep, G.; Venugopal, N. (2010). "Closed system vacuum assisted administration of high dose radio iodine to cancer thyroid patients: NIMS techniqe [sic]". Indian J Nucl Med 25 (1): 34–5. doi:10.4103/0972-3919.63601. PMID 20844671.
- ↑ Luster, M.; Clarke, S. E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W. J. G.; Tennvall, J.; Bombardieri, E. (1 August 2008). "Guidelines for radioiodine therapy of differentiated thyroid cancer". European Journal of Nuclear Medicine and Molecular Imaging 35 (10): 1941–1959. doi:10.1007/s00259-008-0883-1. PMID 18670773.
- ↑ Nuclear medicine in thyroid cancer management: a practical approach.. Vienna: International Atomic Energy Agency. 2009. pp. 1–288. ISBN 978-92-0-113108-9. http://www-pub.iaea.org/books/IAEABooks/7947/Nuclear-Medicine-in-Thyroid-Cancer-Management-A-Practical-Approach.
- ↑ Valentine, J. (June 2004). "ICRP Publication 94: Release of Nuclear Medicine Patients after Therapy with Unsealed Sources". Annals of the ICRP 34 (2): 1–27. doi:10.1016/j.icrp.2004.08.003.
- ↑ "Radioiodine Therapy: Information for Patients". AACE. 2004. http://www.kumc.edu/endocrine/Radioiodine_Therapy.pdf.
- ↑ "Instructions for Receiving Radioactive Iodine Therapy after a Thyroid Cancer Survey". University of Washington Medical Center. http://uwmedicine.washington.edu/PatientCare/MedicalSpecialties/SpecialtyCare/UWMEDICALCENTER/Radiology/instructionsthyroidcancersurvey.htm.
- ↑ "Precautions after Out-patient Radioactive Iodine (I-131) Therapy". Department of Nuclear Medicine McMaster University Medical Centre. http://www.hamiltonhealthsciences.ca/documents/Patient%20Education/I131RadioactiveIodineTherapyHHS-trh.pdf.
- ↑ "Biosafety Manual for Indiana University–Purdue University Indianapolis". Indiana University–Purdue University Indianapolis. May 2002. p. 7. http://www.ehs.iupui.edu/biohaz_manual/biosafety_manual_v0502.pdf.
- ↑ Sutton, Jane (29 January 2007). "Radioactive patients". reuters. https://www.reuters.com/article/health-SP-A/idUSN2633076820070209?pageNumber=2.
- ↑ "Medical isotopes the likely cause of radiation in Ottawa waste". CBC News. 4 February 2009. http://www.cbc.ca/news/canada/medical-isotopes-the-likely-cause-of-radiation-in-ottawa-waste-1.852645.
- ↑ Moser, H.; Rauert, W. (2007). "Isotopic Tracers for Obtaining Hydrologic Parameters". in Aggarwal, Pradeep K.; Gat, Joel R.; Froehlich, Klaus F.. Isotopes in the water cycle : past, present and future of a developing science. Dordrecht: Springer. p. 11. ISBN 978-1-4020-6671-9. https://books.google.com/books?id=XKk6V_IeJbIC&pg=PA11. Retrieved 6 May 2012.
- ↑ Rao, S. M. (2006). "Radioisotopes of hydrological interest". Practical isotope hydrology. New Delhi: New India Publishing Agency. pp. 12–13. ISBN 978-81-89422-33-2. https://books.google.com/books?id=E7TVDVVji0EC&q=isotope%20hydrology%20iodine&pg=PA11. Retrieved 6 May 2012.
- ↑ "Investigating leaks in Dams & Reservoirs". IAEA.org. http://www.iaea.org/technicalcooperation/documents/sheet20dr.pdf.
- ↑ Araguás, Luis Araguás; Plata Bedmar, Antonio (2002). "Artificial radioactive tracers". Detection and prevention of leaks from dams. Taylor & Francis. pp. 179–181. ISBN 978-90-5809-355-4. https://books.google.com/books?id=FXB-HMzfBnkC&pg=PA179. Retrieved 6 May 2012.
- ↑ Reis, John C. (1976). "Radioactive materials". Environmental Control in Petroleum Engineering. Gulf Professional Publishers. p. 55. ISBN 978-0-88415-273-6. https://books.google.com/books?id=XAseQ35m2OYC&pg=PA54.
- ↑ McKinley, R. M. (1994). "Radioactive tracer surveys". Temperature, radioactive tracer, and noise logging for injection well integrity. Washington: U.S. Environmental Protection Agency. http://www.epa.gov/ogwdw/uic/pdfs/Historical/techguide_uic_temp_tracer__noise_logging_1994.pdf. Retrieved 6 May 2012.
- ↑ Schlumberger Ltd. "Radioactive-tracer log". Schlumberger.com. http://www.glossary.oilfield.slb.com/Display.cfm?Term=radioactive-tracer%20log.
- ↑ Scott, George L., "Method for monitoring the hydraulic fracturing of a subterranean formation", US patent 5635712, published 1997-06-03
- ↑ Fertl, Walter H., "Method and apparatus for evaluating multiple stage fracturing or earth formations surrounding a borehole", US patent 4415805, published 1983-11-15
- ↑ Scott, George L., "System and method for monitoring fracture growth during hydraulic fracture treatment", US patent 5441110, published 1995-08-15
External links
- "ANL factsheet". http://www.ead.anl.gov/pub/doc/iodine.pdf.
- RadiologyInfo – The radiology information resource for patients: Radioiodine (I −131) Therapy
- Case Studies in Environmental Medicine: Radiation Exposure from Iodine 131
- Sensitivity of Personal Homeland Security Radiation Detectors to Medical Radionuclides and Implications for Counseling of Nuclear Medicine Patients
- NLM Hazardous Substances Databank – Iodine, Radioactive
| Lighter: 130I |
Iodine-131 is an isotope of iodine |
Heavier: 132I |
| Decay product of: '131Te (β−)' |
Decay chain of iodine-131 |
Decays to: '131Xe (β−)' |
 |
