Chemistry:Meso-octamethylporphyrinogen
| |
| Names | |
|---|---|
| IUPAC name
5,5,10,10,15,15,20,20-octamethyl-21,22,23,24-tetrahydroporphyrin
| |
| Other names
octamethylcalix(4)pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 365398 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 2142403 | |
PubChem CID
|
|
| |
| |
| Properties | |
| C28H36N4 | |
| Molar mass | 428.624 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
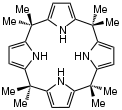
Meso-octamethylporphyrinogen, usually referred to simply as octamethylporphyrinogen, is an organic compound with the formula (Me2C-C4H2NH)4 (Me = CH3. It is one of the simplest porphyrinogens, a family of compounds that occur as intermediates in the biosynthesis of hemes and chlorophylls. In contrast to those rings, porphyrinogens are colorless since they lack extended conjugation. The prefix meso-octamethyl indicates that eight methyl groups are located on the carbon centers that interconnect the four pyrrole rings. Also unlike porphyrins, the porphyrinogens are highly ruffled. The compound is made by condensation of pyrrole with acetone.[1]
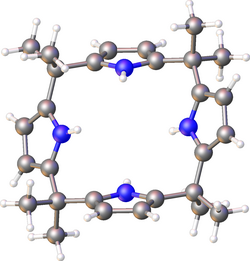
The pyrrolic N-H centers of ctamethylporphyrinogen can be deprotonated, and the resulting tetraanion functions as a tetradentate ligand for a variety of metal ions.[3]
References
- ↑ Sessler, Jonathan L.; Anzenbacher, Pavel Jr.; Miyaji, Hidekazu; Jursikova, Karolina; Bleasdale, Ellen R.; Gale, Philip A. (2000). "Modified Calix[4]pyrroles". Industrial & Engineering Chemistry Research 39 (10): 3471–3478. doi:10.1021/ie000102y.
- ↑ Allen, William E.; Gale, Philip A.; Brown, Christopher T.; Lynch, Vincent M.; Sessler, Jonathan L. (1996). "Binding of Neutral Substrates by Calix[4]pyrroles". Journal of the American Chemical Society 118 (49): 12471–12472. doi:10.1021/ja9632217.
- ↑ Bachmann, Julien; Hodgkiss, Justin M.; Young, Elizabeth R.; Nocera, Daniel G. (2007). "Ground- and Excited-State Reactivity of Iron Porphyrinogens". Inorganic Chemistry 46 (3): 607–609. doi:10.1021/ic0616636. PMID 17256999.

