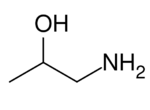
Chemistry:1-Amino-2-propanol
| |
| Names | |
|---|---|
| IUPAC name
1-Aminopropan-2-ol
| |
| Other names
Isopropanolamine
Monoisopropanolamine MIPA; Threamine | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | MIPA |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.111 g·mol−1 |
| Appearance | liquid |
| Odor | ammonia-like |
| Density | 0.973 g/mL (18 °C) [1] |
| Melting point | 1.74 °C (35.13 °F; 274.89 K) |
| Boiling point | 159.46 °C (319.03 °F; 432.61 K) |
| soluble | |
| Solubility | soluble in alcohol, ether, acetone, benzene, CCl4 |
Refractive index (nD)
|
1.4479 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 77 °C (171 °F; 350 K) |
| 374 °C (705 °F; 647 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4.26 g/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Amino-2-propanol (isopropanolamine) is a chemical compound with the formula C3H9NO. It is an amino alcohol. The term "isopropanolamine" may also refer more generally to the additional homologs diisopropanolamine (DIPA) and triisopropanolamine (TIPA).
It can be prepared by the addition of aqueous ammonia to propylene oxide.
Applications
The isopropanolamines are basic chemicals that can be used in many applications to achieve basicity, buffering and alkalinity objectives. They are good solubilizers of oil and fat, so they are used to neutralize fatty acids and sulfonic acid-based surfactants.
1-Amino-2-propanol is typically used in metal working fluid, waterborne coating, personal care products, and in the production of titanium dioxide and polyurethanes.[2]
1-Amino-2-propanol is an intermediate in the synthesis of a variety of pharmaceutical drugs and is the very basic building block of the opioid methadone.
The chemical derivative 1-dimethylamino-2-propanol can be synthesized via Eschweiler-Clarke methylation of 1-amino-2-propanol using formic acid and formaldehyde.[citation needed]
References
- ↑ Amino-2-propanol at Sigma-Aldrich
- ↑ "Monoisopropanolamine". Nanjing HBL International. http://www.hbltrade.com/pid10029823/Monoisopropanolamine.htm. Retrieved October 24, 2012.


