Chemistry:Hydroxylammonium chloride
| |
| |

| |
| Names | |
|---|---|
| Other names
Hydroxylamine hydrochloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| ClH4NO | |
| Molar mass | 69.49 g·mol−1 |
| Appearance | white crystalline solid |
| Density | 1.67 g/cm3 |
| Melting point | 155 to 157 °C (311 to 315 °F; 428 to 430 K) decomposes |
| Conjugate base | Hydroxylamine |
| Hazards | |
| GHS pictograms |     
|
| GHS Signal word | Danger |
| H290, H301, H302, H312, H315, H317, H319, H351, H373, H400 | |
| P201, P202, P234, P260, P261, P264, P270, P272, P273, P280, P281, P301+310, P301+312, P302+352, P305+351+338, P308+313, P312, P314, P321, P322, P330, P332+313, P333+313, P337+313, P362 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
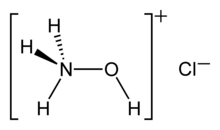
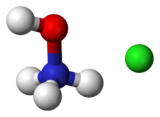
Hydroxylammonium chloride is a chemical compound with the formula [NH
3OH]+
Cl−
. It is the hydrochloric acid salt of hydroxylamine (NH
2OH). Hydroxylamine is a biological intermediate in nitrification (biological oxidation of ammonia with oxygen into nitrite) and in anammox (biological oxidation of nitrite and ammonium into dinitrogen gas) which are important in the nitrogen cycle in soil and in wastewater treatment plants.
Applications
Hydroxylammonium chloride is used in organic synthesis for preparation of oximes and hydroxamic acids from carboxylic acids, N- and O- substituted hydroxylamines, and addition reactions of carbon-carbon double bond.
During the acetyl bromide method of extracting lignin from lignocellulosic biomass, hydroxylammonium chloride can be used to remove bromine and polybromide from the solution.
In surface treatments, it is used in the preparation of anti-skinning agents, corrosion inhibitors, and cleaner additives. It is also a starting material for pharmaceuticals and agrochemicals manufacturing. In the rubber and plastics industries, it is an antioxidant, vulcanization accelerator, and radical scavenger.
It is also used as a fixative for textile dyes, auxiliary in some dyeing processes, as a metal extraction and flotation aid, as an antioxidant in fatty acids and soaps, and as a color stabilizer and emulsion additive in color films.
It is also used in analytic chemistry in the analysis of iron in the water combined with α,α-dipyridyl. The hydroxylammonium chloride transforms all the iron in Fe2+, that then forms a coordination complex with the dipyridyl.
References
- Fukushima, R.S.; Dehority, B.A.; Loerch, S.C. (1 January 1991). "Modification of a colorimetric analysis for lignin and its use in studying the inhibitory effects of lignin on forage digestion by ruminal microorganisms". J. Anim. Sci. 69 (1): 295–304. doi:10.2527/1991.691295x. PMID 2005024. http://jas.fass.org/cgi/content/abstract/69/1/295.
- Elstner, E.F.; Heupel, A. (February 1976). "Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase". Anal. Biochem. 70 (2): 616–20. doi:10.1016/0003-2697(76)90488-7. PMID 817618.
 |


