Chemistry:Hydroxylammonium sulfate
| |
| |
| |
| Names | |
|---|---|
| Other names
Hydroxylamine sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2865 |
| |
| |
| Properties | |
| H8N2O6S | |
| Molar mass | 164.14 g/mol |
| Appearance | white crystalline to fine product, slightly hygroscopic |
| Density | 1.88 g/cm3 |
| Melting point | 120 °C (248 °F; 393 K) decomposes |
| 58.7 g/100 ml (20 °C) | |
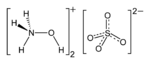
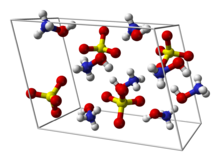
| Structure[1] | |
| Monoclinic | |
| P21/c | |
α = 90°, β = 106.93±0.03°, γ = 90°
| |
Formula units (Z)
|
4 |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |    
|
| GHS Signal word | Warning |
| H290, H302, H312, H315, H317, H319, H351, H373, H400, H412 | |
| P201, P202, P234, P260, P261, P264, P270, P272, P273, P280, P281, P301+312, P302+352, P305+351+338, P308+313, P312, P314, P321, P322, P330, P332+313, P333+313, P337+313, P362, P363 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Hydroxylammonium nitrate Hydroxylammonium chloride |
Other cations
|
Ammonium sulfate Hydrazinium sulfate |
Related compounds
|
Hydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxylammonium sulfate (NH3OH)2SO4, is the sulfuric acid salt of hydroxylamine. It is primarily used as an easily handled form of hydroxylamine, which is explosive when pure.
Synthesis
Hydroxylammonium sulfate is prepared industrially via the Raschig hydroxylamine process, which involves the reduction of nitrite with bisulfite. This initially gives hydroxylamine disulfonate, which is hydrolysed to hydroxylammonium sulfate:[2] It can also be obtained by the acid-base reaction of hydroxylamine with sulfuric acid:
- 2NH2OH(aq) + H2SO4(aq) → (NH3OH)2SO4(aq)
Applications
Hydroxylammonium sulfate is used in organic synthesis to convert aldehydes and ketones to oximes, carboxylic acids and their derivatives (e.g. esters) to hydroxamic acids, isocyanates to N-hydroxyureas and nitriles to amidoximes. Hydroxylammonium sulfate is also used to generate hydroxylamine-O-sulfonic acid from oleum or chlorosulfuric acid.
Hydroxylammonium sulfate is used in the production of anti-skinning agents, pharmaceuticals, rubber, textiles, plastics and detergents. It is a radical scavenger that terminates radical polymerization reactions and serves as an antioxidant in natural rubber. (NH3OH)2SO4 is a starting material for some insecticides, herbicides and growth regulators. It is used in photography as a stabiliser for colour developers and as an additive in photographic emulsions in colour film.
Decomposition
At 120 °C, hydroxylammonium sulfate begins to decompose to sulfur trioxide, nitrous oxide, water, and ammonia[dubious ]:
- 2(NH3OH)2SO4 → 2SO3 + N2O + 2NH3 + 5H2O
The reaction is exothermic above 138 °C, and is most exothermic at 177 °C.[3] Metals (especially copper, its alloys and its salts) catalyse the decomposition of hydroxylammonium sulfate. The instability of this compound is mainly due to the hydroxylammonium ion's weak nitrogen to oxygen single bond.
References
- ↑ Mirceva, A.; Golic, L. (15 May 1995). "Hydroxylammonium Sulfate". Acta Crystallographica Section C Crystal Structure Communications 51 (5): 798–800. doi:10.1107/S0108270194013351.
- ↑ Oblath, S. B.; Markowitz, S. S.; Novakov, T.; Chang, S. G. (December 1982). "Kinetics of the initial reaction of nitrite ion in bisulfite solutions". The Journal of Physical Chemistry 86 (25): 4853–4857. doi:10.1021/j100222a005. https://escholarship.org/content/qt7j51f662/qt7j51f662.pdf?t=n4fudf.
- ↑ BASF hydroxylammonium sulfate product page
External links
 |


