Chemistry:Methylenecyclopropane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methylidenecyclopropane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 4H 6 | |
| Molar mass | 54.09 |
| Density | 0.8 g/cm3 |
| Boiling point | 9 to 12 °C (48 to 54 °F; 282 to 285 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
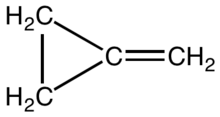
Methylenecyclopropane is an organic compound with the formula (CH
2)
2C=CH
2. It is a hydrocarbon which, as the name suggests, is derived from the addition of a methylene (=CH
2) substituent to a cyclopropane ring. It is a colourless, easily condensed gas that is used as a reagent in organic synthesis.
Synthesis
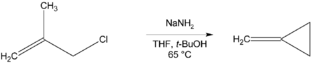
Methylenecyclopropane can be synthesized via an intramolecular cyclisation reaction from methallyl chloride by treatment with a strong base such sodium amide—sodium tert-butoxide (yield 43%)[1] or sodium bis(trimethylsilyl)amide with further treatment by sodium tert-butoxide (yield 72%).[2] Sodium tert-butoxide is used to isomerize byproduct 1-methylcyclopropene into methylenecyclopropane.
Reactions
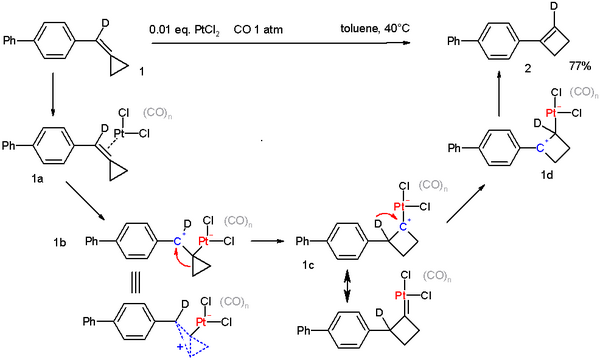
Being a strained and unsaturated molecule methylenecyclopropane undergoes many reactions, especially in the presence of metal catalysts.[3] For example, methylenecyclopropanes can be converted to cyclobutenes in the presence of a Platinum catalyst.[4] This can be considered similar to the ring expansion seen in vinylcyclopropane rearrangements
Substituted methylenecyclopropanes can also be involved in trimethylenemethane cycloaddition reactions.
See also
References
- ↑ Salaun, J. R.; Champion, J.; Conia, J. M. (1977). "Cyclobutanone from Methylenecyclopropane via Oxaspiropentane". Organic Syntheses 57: 36. doi:10.15227/orgsyn.057.0036.
- ↑ Binger, Paul; Brinkmann, Axel; Wedemann, Petra (2002). "Highly Efficient Synthesis of Methylenecyclopropane" (in en). Synthesis (10): 1344–1346. doi:10.1055/s-2002-33122. ISSN 0039-7881. http://www.thieme-connect.de/DOI/DOI?10.1055/s-2002-33122.
- ↑ Nakamura, I.; Yamamoto, Y. (2002). "Transition Metal-Catalyzed Reactions of Methylenecyclopropanes". Advanced Synthesis and Catalysis 344 (2): 111–129. doi:10.1002/1615-4169(200202)344:2<111::AID-ADSC111>3.0.CO;2-0.
- ↑ PtCl2-Catalyzed Rearrangement of Methylenecyclopropanes Alois Fürstner and Christophe Aïssa J. Am. Chem. Soc.; 2006; 128(19) pp 6306 -6307; Abstract
 |