Astronomy:Greenhouse effect
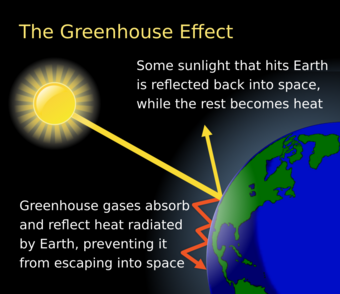
The greenhouse effect is the process by which radiation from a planet's atmosphere warms the planet's surface to a temperature above what it would be without this atmosphere.[1][2]
Radiatively active gases (i.e., greenhouse gases) in a planet's atmosphere radiate energy in all directions. Part of this radiation is directed towards the surface, thus warming it.[3] Similarly, aerosols have radiatively active effects.[4] The intensity of downward radiation – that is, the strength of the greenhouse effect – depends on the amount of greenhouse gases and aerosols that the atmosphere contains. The temperature rises until the intensity of upward radiation from the surface, thus cooling it, balances the downward energy flow.[5]
Earth's natural greenhouse effect is critical to supporting life and initially was a precursor to life moving out of the ocean onto land. Human activities, mainly the burning of fossil fuels and clearcutting of forests, have increased the greenhouse effect and caused global warming.[6]
The planet Venus experienced a runaway greenhouse effect, resulting in an atmosphere which is 96% carbon dioxide, and a surface atmospheric pressure roughly the same as found 900 m (3,000 ft) underwater on Earth. Venus may have had water oceans, but they would have boiled off as the mean surface temperature rose to the current 735 K (462 °C; 863 °F).[7][8][9]
The term greenhouse effect is a slight misnomer, in the sense that physical greenhouses warm via a different mechanism. The greenhouse effect as an atmospheric mechanism functions through radiative heat loss[10] while a traditional greenhouse as a built structure blocks convective heat loss.[2] The result, however, is an increase in temperature in both cases.[11][12]
History
The existence of the greenhouse effect, while not named as such, was proposed by Joseph Fourier in 1824.[13] The argument and the evidence were further strengthened by Claude Pouillet in 1827 and 1838. John Tyndall was the first to measure the infrared absorption and emission of various gases and vapors. From 1859 onwards, he showed that the effect was due to a very small proportion of the atmosphere, with the main gases having no effect, and was largely due to water vapor, though small percentages of hydrocarbons and carbon dioxide had a significant effect.[14] The effect was more fully quantified by Svante Arrhenius in 1896, who made the first quantitative prediction of global warming due to a hypothetical doubling of atmospheric carbon dioxide.[15] However, the term "greenhouse" was not used to refer to this effect by any of these scientists; the term was first used in this way by Nils Gustaf Ekholm in 1901.[16][17]
Description
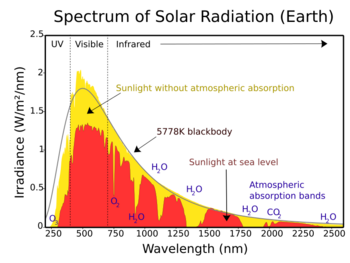
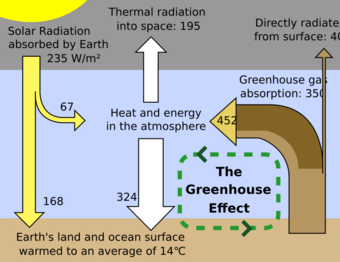
Earth receives energy from the Sun in the form of ultraviolet, visible, and near-infrared radiation. About 26% of the incoming solar energy is reflected back to space by the atmosphere and clouds, and 19% is absorbed by the atmosphere and clouds. Most of the remaining energy is absorbed at the surface of Earth. Because the Earth's surface is colder than the Sun, it radiates at wavelengths that are much longer than the wavelengths that were absorbed. Most of this thermal radiation is absorbed by the atmosphere and warms it. The atmosphere also gains heat by sensible and latent heat fluxes from the surface. The atmosphere radiates energy both upwards and downwards; the part radiated downwards is absorbed by the surface of Earth. This leads to a higher equilibrium temperature than if the atmosphere did not radiate.
An ideal thermally conductive blackbody at the same distance from the Sun as Earth would have a temperature of about 5.3 °C (41.5 °F). However, because Earth reflects about 30%[18][19] of the incoming sunlight, this idealized planet's effective temperature (the temperature of a blackbody that would emit the same amount of radiation) would be about −18 °C (0 °F).[20][21] The surface temperature of this hypothetical planet is 33 °C (59 °F) below Earth's actual surface temperature of approximately 14 °C (57 °F).[22] The greenhouse effect is the contribution of greenhouse gases and aerosols to this difference.[clarification needed]
Details
The idealized greenhouse model is a simplification. In reality, the atmosphere near the Earth's surface is largely opaque to thermal radiation and most heat loss from the surface is by convection. However radiative energy losses become increasingly important higher in the atmosphere, largely because of the decreasing concentration of water vapor, an important greenhouse gas. Rather than the surface itself, it is more realistic to think of the greenhouse effect as applying to a layer in the mid-troposphere, which is effectively coupled to the surface by a lapse rate. A simple picture also assumes a steady state, but in the real world, the diurnal cycle, as well as the seasonal cycle and weather disturbances, complicate matters. Solar heating applies only during daytime. During the night, the atmosphere cools somewhat, but not greatly, because its emissivity is low. Diurnal temperature changes decrease with height in the atmosphere.
Within the region where radiative effects are important, the description given by the idealized greenhouse model becomes realistic. Earth's surface, warmed to an "effective temperature" around −18 °C (0 °F), radiates long-wavelength, infrared heat in the range of 4–100 μm.[23] At these wavelengths, greenhouse gases that were largely transparent to incoming solar radiation are more absorbent.[23] Each layer of the atmosphere with greenhouse gases absorbs some of the heat being radiated upwards from lower layers. It reradiates in all directions, both upwards and downwards; in equilibrium (by definition) the same amount as it has absorbed. This results in more warmth below. Increasing the concentration of the gases increases the amount of absorption and re-radiation, and thereby further warms the layers and ultimately the surface below.[21]
Greenhouse gases—including most diatomic gases with two different atoms (such as carbon monoxide, CO) and all gases with three or more atoms—are able to absorb and emit infrared radiation. Though more than 99% of the dry atmosphere is IR transparent (because the main constituents—N2, O2, and Ar—are not able to directly absorb or emit infrared radiation), intermolecular collisions cause the energy absorbed and emitted by the greenhouse gases to be shared with the other, non-IR-active, gases.
Greenhouse gases
By their percentage contribution to the greenhouse effect on Earth the four major gases are:[24][25]
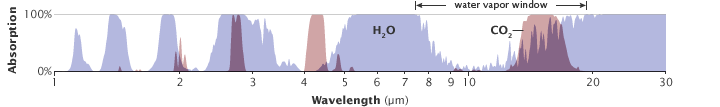
- water vapor, 36–70%
- carbon dioxide, 9–26%
- methane, 4–9%
- ozone, 3–7%
It is not possible to assign a specific percentage to each gas because the absorption and emission bands of the gases overlap (hence the ranges given above). Clouds also absorb and emit infrared radiation and thus affect the radiative properties of the atmosphere.[25]
Role in climate change
Strengthening of the greenhouse effect through human activities is known as the enhanced (or anthropogenic) greenhouse effect.[27] This increase in radiative forcing from human activity has been observed directly[28][29] and is attributable mainly to increased atmospheric carbon dioxide levels.[30] According to the 2014 Assessment Report from the Intergovernmental Panel on Climate Change, "atmospheric concentrations of carbon dioxide, methane and nitrous oxide are unprecedented in at least the last 800,000 years. Their effects, together with those of other anthropogenic drivers, have been detected throughout the climate system and are extremely likely to have been the dominant cause of the observed warming since the mid-20th century'".[31]
CO
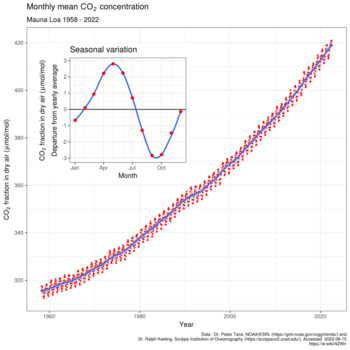
2 is produced by fossil fuel burning and other activities such as cement production and tropical deforestation.[32] Measurements of CO
2 from the Mauna Loa observatory show that concentrations have increased from about 313 parts per million (ppm)[33] in 1960, passing the 400 ppm milestone on May 9, 2013.[34] The current observed amount of CO
2 exceeds the geological record maxima (≈300 ppm) from ice core data.[35] The effect of combustion-produced carbon dioxide on the global climate, a special case of the greenhouse effect first described in 1896 by Svante Arrhenius, has also been called the Callendar effect.
Over the past 800,000 years,[36] ice core data shows that carbon dioxide has varied from values as low as 180 ppm to the pre-industrial level of 270 ppm.[37] Paleoclimatologists consider variations in carbon dioxide concentration to be a fundamental factor influencing climate variations over this time scale.[38][39]
Real greenhouses
The "greenhouse effect" of the atmosphere is named by analogy to greenhouses which become warmer in sunlight. However, a greenhouse is not primarily warmed by the "greenhouse effect".[40] "Greenhouse effect" is actually a misnomer since heating in the usual greenhouse is due to the reduction of convection,[12] while the "greenhouse effect" works by preventing absorbed heat from leaving the structure through radiative transfer.
A greenhouse is built of any material that passes sunlight: usually glass or plastic. The sun warms the ground and contents inside just like the outside, and these then warm the air. Outside, the warm air near the surface rises and mixes with cooler air aloft, keeping the temperature lower than inside, where the air continues to heat up because it is confined within the greenhouse. This can be demonstrated by opening a small window near the roof of a greenhouse: the temperature will drop considerably. It was demonstrated experimentally (R. W. Wood, 1909) that a (not heated) "greenhouse" with a cover of rock salt (which is transparent to infrared) heats up an enclosure similarly to one with a glass cover.[11] Thus greenhouses work primarily by preventing convective cooling.[10]
Heated greenhouses are yet another matter: as they have an internal source of heating, it is desirable to minimize the amount of heat leaking out by radiative cooling. This can be done through the use of adequate glazing.[41]
It is possible in theory to build a greenhouse that lowers its thermal emissivity during dark hours;[42] such a greenhouse would trap heat by two different physical mechanisms, combining multiple greenhouse effects, one of which more closely resembles the atmospheric mechanism, rendering the misnomer debate moot.
Related effects
Anti-greenhouse effect
The anti-greenhouse effect is a mechanism similar and symmetrical to the greenhouse effect: in the greenhouse effect, the atmosphere lets radiation in while not letting thermal radiation out, thus warming the body surface; in the anti-greenhouse effect, the atmosphere keeps radiation out while letting thermal radiation out, which lowers the equilibrium surface temperature. Such an effect has been proposed for Saturn's moon Titan.[43]
Runaway greenhouse effect
A runaway greenhouse effect occurs if positive feedbacks lead to the evaporation of all greenhouse gases into the atmosphere.[44] A runaway greenhouse effect involving carbon dioxide and water vapor has long ago been hypothesized to have occurred on Venus,[45] this idea is still largely accepted.[46]
Bodies other than Earth
The 'greenhouse effect' on Venus is particularly large for several reasons:
- It is nearer to the Sun than Earth by about 30%.
- Its very dense atmosphere consists mainly of carbon dioxide.[47]
"Venus experienced a runaway greenhouse in the past, and we expect that Earth will in about 2 billion years as solar luminosity increases".[48]
Titan is a body with both a greenhouse effect and an anti-greenhouse effect. The presence of N2, CH4, and H2 in the atmosphere contribute to a greenhouse effect, increasing the surface temperature by 21K over the expected temperature of the body with no atmosphere. The existence of a high-altitude haze, which absorbs wavelengths of solar radiation but is transparent to infrared, contribute to an anti-greenhouse effect of approximately 9K. The net effect of these two phenomena result is a net warming of 21K- 9K= 12K, so Titan is 12 K warmer than it would be if there were no atmosphere.[49][50]
See also
- Top contributors to greenhouse gas emissions
- Climate tipping point
References
- ↑ "Annex III Glossary". Intergovernmental Panel on Climate Change. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_AnnexIII_FINAL.pdf.
- ↑ 2.0 2.1 A concise description of the greenhouse effect is given in the Intergovernmental Panel on Climate Change Fourth Assessment Report, "What is the Greenhouse Effect?" FAQ 1.3 – AR4 WGI Chapter 1: Historical Overview of Climate Change Science, IIPCC Fourth Assessment Report, Chapter 1, page 115: "To balance the absorbed incoming [solar] energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect."
Schneider, Stephen H. (2001). "Global Climate Change in the Human Perspective". in Bengtsson, Lennart O.; Hammer, Claus U.. Geosphere-biosphere Interactions and Climate. Cambridge University Press. pp. 90–91. ISBN 978-0-521-78238-8. https://books.google.com/books?id=MsASkmCPDpcC&pg=PA90.
Claussen, E.; Cochran, V.A.; Davis, D.P., eds (2001). "Global Climate Data". Climate Change: Science, Strategies, & Solutions. University of Michigan. p. 373. ISBN 978-9004120242. https://books.google.com/books?id=g85OJ76ufJoC&pg=PA373.
Allaby, A.; Allaby, M. (1999). A Dictionary of Earth Sciences. Oxford University Press. p. 244. ISBN 978-0-19-280079-4. https://archive.org/details/dictionaryofeart00alla/page/244. - ↑ Vaclav Smil (2003). The Earth's Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN 978-0-262-69298-4. https://books.google.com/books?id=8ntHWPMUgpMC&pg=PA107.
- ↑ Lacis, Andrew; Hansen, James; Sato, Makiko (1992). "Climate forcing by stratospheric aerosols" (in en). Geophysical Research Letters 19 (15): 1607–1610. doi:10.1029/92GL01620. ISSN 1944-8007. https://onlinelibrary.wiley.com/doi/abs/10.1029/92GL01620.
- ↑ Rebecca, Lindsey (2009-01-14). "Climate and Earth's Energy Budget : Feature Articles". https://earthobservatory.nasa.gov/features/EnergyBalance/page1.php.
- ↑ IPCC AR4 WG1 (2007), Solomon, S.; Qin, D.; Manning, M. et al., eds., Climate Change 2007: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, ISBN 978-0-521-88009-1, https://archive.ipcc.ch/publications_and_data/ar4/wg1/en/faq-1-3.html (pb: ISBN:978-0-521-70596-7)
- ↑ Hashimoto, G. L.; Roos-Serote, M.; Sugita, S.; Gilmore, M. S.; Kamp, L. W.; Carlson, R. W.; Baines, K. H. (2008). "Felsic highland crust on Venus suggested by Galileo Near-Infrared Mapping Spectrometer data". Journal of Geophysical Research: Planets 113 (E9): E00B24. doi:10.1029/2008JE003134. Bibcode: 2008JGRE..113.0B24H.
- ↑ David Shiga (10 October 2007). "Did Venus's ancient oceans incubate life?". New Scientist. https://www.newscientist.com/article/dn12769-did-venuss-ancient-oceans-incubate-life.html.
- ↑ Jakosky, Bruce M. (1999). "Atmospheres of the Terrestrial Planets". in Beatty, J. Kelly; Petersen, Carolyn Collins; Chaikin, Andrew. The New Solar System (4th ed.). Boston: Sky Publishing. pp. 175–200. ISBN 978-0-933346-86-4. OCLC 39464951.
- ↑ 10.0 10.1 Schroeder, Daniel V. (2000). An introduction to thermal physics. Addison-Wesley. pp. 305–7. ISBN 978-0-321-27779-4. "... this mechanism is called the greenhouse effect, even though most greenhouses depend primarily on a different mechanism (namely, limiting convective cooling)."
- ↑ 11.0 11.1 Wood, R.W. (1909). "Note on the Theory of the Greenhouse". Philosophical Magazine 17 (98): 319–320. doi:10.1080/14786440208636602. http://www.wmconnolley.org.uk/sci/wood_rw.1909.html. "When exposed to sunlight the temperature rose gradually to 65 °C., the enclosure covered with the salt plate keeping a little ahead of the other because it transmitted the longer waves from the Sun, which were stopped by the glass. In order to eliminate this action the sunlight was first passed through a glass plate." "it is clear that the rock-salt plate is capable of transmitting practically all of it, while the glass plate stops it entirely. This shows us that the loss of temperature of the ground by radiation is very small in comparison to the loss by convection, in other words that we gain very little from the circumstance that the radiation is trapped.".
- ↑ 12.0 12.1 Oort, Abraham H.; Peixoto, José Pinto (1992). Physics of climate. New York: American Institute of Physics. ISBN 978-0-88318-711-1. "...the name water vapor-greenhouse effect is actually a misnomer since heating in the usual greenhouse is due to the reduction of convection"
- ↑ Fourier, J. (1824). "Remarques Generales sur les Temperatures Du Globe Terrestre et des Espaces Planetaires" (in fr). Annales de Chimie et de Physique 27: 136–167. https://books.google.com/books?id=1Jg5AAAAcAAJ&pg=PA136.
- ↑ John Tyndall, Heat considered as a Mode of Motion (500 pages; year 1863, 1873)
- ↑ Held, Isaac M.; Soden, Brian J. (November 2000). "Water Vapor Feedback and Global Warming". Annual Review of Energy and the Environment 25: 441–475. doi:10.1146/annurev.energy.25.1.441.
- ↑ Easterbrook, Steve (18 August 2015). "Who first coined the term "Greenhouse Effect"?". http://www.easterbrook.ca/steve/2015/08/who-first-coined-the-term-greenhouse-effect/.
- ↑ Ekholm N (1901). "On The Variations Of The Climate Of The Geological And Historical Past And Their Causes". Quarterly Journal of the Royal Meteorological Society 27 (117): 1–62. doi:10.1002/qj.49702711702. Bibcode: 1901QJRMS..27....1E.
- ↑ "NASA Earth Fact Sheet". Nssdc.gsfc.nasa.gov. http://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html.
- ↑ Jacob, Daniel J. (1999). "7. The Greenhouse Effect". Introduction to Atmospheric Chemistry. Princeton University Press. ISBN 978-1400841547. http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html.
- ↑ "Solar Radiation and the Earth's Energy Balance". Eesc.columbia.edu. http://eesc.columbia.edu/courses/ees/climate/lectures/radiation/.
- ↑ 21.0 21.1 Intergovernmental Panel on Climate Change Fourth Assessment Report. Chapter 1: Historical overview of climate change science page 97
- ↑ The elusive "absolute surface air temperature," see GISS discussion
- ↑ 23.0 23.1 Mitchell, John F. B. (1989). "The "Greenhouse" effect and Climate Change". Reviews of Geophysics 27 (1): 115–139. doi:10.1029/RG027i001p00115. Bibcode: 1989RvGeo..27..115M. http://astrosun2.astro.cornell.edu/academics/courses/astro202/Mitchell_GRL89.pdf. Retrieved 2008-03-23.
- ↑ "Water vapour: feedback or forcing?". RealClimate. 6 April 2005. http://www.realclimate.org/index.php?p=142.
- ↑ 25.0 25.1 Kiehl, J.T.; Trenberth, Kevin E. (February 1997). "Earth's Annual Global Mean Energy Budget". Bulletin of the American Meteorological Society 78 (2): 197–208. doi:10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2. Bibcode: 1997BAMS...78..197K. http://www.atmo.arizona.edu/students/courselinks/spring04/atmo451b/pdf/RadiationBudget.pdf. Retrieved 2006-05-01.
- ↑ "NASA: Climate Forcings and Global Warming". January 14, 2009. http://earthobservatory.nasa.gov/Features/EnergyBalance/page7.php.
- ↑ "Enhanced greenhouse effect — Glossary". Nova. Australian Academy of Scihuman impact on the environment. 2006. http://www.science.org.au/nova/016/016glo.htm.
- ↑ Robert McSweeney (2015-02-25). "New study directly measures greenhouse effect at Earth's surface". Carbon Brief. https://www.carbonbrief.org/new-study-directly-measures-greenhouse-effect-at-earths-surface.
- ↑ "Direct observations confirm that humans are throwing Earth's energy budget off balance". Science X. 2021-03-26. https://phys.org/news/2021-03-humans-earth-energy.html.
- ↑ "Enhanced Greenhouse Effect". Ace.mmu.ac.uk. http://www.ace.mmu.ac.uk/eae/Global_Warming/Older/Enhanced_Greenhouse_Effect.html.
- ↑ "Synthesis Report: Summary for Policymakers". IPCC Fifth Assessment Report. p. 4. http://www.ipcc.ch/pdf/assessment-report/ar5/syr/AR5_SYR_FINAL_SPM.pdf.
- ↑ IPCC Fourth Assessment Report, Working Group I Report "The Physical Science Basis" Chapter 7
- ↑ "Atmospheric Carbon Dioxide – Mauna Loa". NOAA. http://www.esrl.noaa.gov/gmd/ccgg/trends/co2_data_mlo.html.
- ↑ "Climate Milestone: Earth's CO2 Level Passes 400 ppm". National Geographic. 2013-05-12. http://news.nationalgeographic.com/news/energy/2013/05/130510-earth-co2-milestone-400-ppm/. Retrieved 2017-12-10.
- ↑ Hansen J. (February 2005). "A slippery slope: How much global warming constitutes "dangerous anthropogenic interference"?". Climatic Change 68 (333): 269–279. doi:10.1007/s10584-005-4135-0. Bibcode: 2005ClCh...68..269H. https://zenodo.org/record/1232798.
- ↑ "Deep ice tells long climate story". BBC News. 2006-09-04. http://news.bbc.co.uk/2/hi/science/nature/5314592.stm.
- ↑ Hileman B (2005-11-28). "Ice Core Record Extended". Chemical & Engineering News 83 (48): 7. doi:10.1021/cen-v083n048.p007. http://pubs.acs.org/cen/news/83/i48/8348notw1.html.
- ↑ Bowen, Mark (2006). Thin Ice: Unlocking the Secrets of Climate in the World's Highest Mountains. Owl Books. ISBN 978-1429932707. https://books.google.com/books?id=HGIcabCZHVUC.
- ↑ Temperature change and carbon dioxide change, U.S. National Oceanic and Atmospheric Administration
- ↑ Brian Shmaefsky (2004). Favorite demonstrations for college science: an NSTA Press journals collection. NSTA Press. p. 57. ISBN 978-0-87355-242-4. https://books.google.com/books?id=L4jtv2mX0iQC&pg=PA57.
- ↑ Kurpaska, Sławomir (2014). "Energy effects during using the glass with different properties in a heated greenhouse". Technical Sciences 17 (4): 351–360. http://uwm.edu.pl/wnt/technicalsc/tech_17_4/b04.pdf.
- ↑ Darrin, Ann (2000). "Variable emissivity through MEMS technology". ITHERM 2000. The Seventh Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (Cat. No.00CH37069). pp. 264–270. doi:10.1109/ITHERM.2000.866834. ISBN 0-7803-5912-7. https://ieeexplore.ieee.org/document/866834. Retrieved 7 January 2021. "Specialized thermal control coatings, which can passively or actively adjust their emissivity offer an attractive solution to these [spacecraft] design challenges."
- ↑ "Titan: Greenhouse and Anti-greenhouse". Astrobiology Magazine – earth science – evolution distribution Origin of life universe – life beyond :: Astrobiology is study of earth. http://www.astrobio.net/news/modules.php?op=modload&name=News&file=article&sid=1762&mode=thread&order=0&thold=0.
- ↑ Kasting, James F. (1991). "Runaway and moist greenhouse atmospheres and the evolution of Earth and Venus.". Commission on Engineering and Technical Systems (CETS). pp. 234–245. http://books.nap.edu/openbook.php?record_id=1790&page=234. Retrieved 9 April 2017.
- ↑ Rasool, I.; De Bergh, C. (June 1970). "The Runaway Greenhouse and the Accumulation of CO2 in the Venus Atmosphere". Nature 226 (5250): 1037–9. doi:10.1038/2261037a0. PMID 16057644. Bibcode: 1970Natur.226.1037R. http://pubs.giss.nasa.gov/docs/1970/1970_Rasool_DeBergh.pdf.
- ↑ McCarthy, Michael Cabbage and Leslie. "NASA climate modeling suggests Venus may have been habitable". https://climate.nasa.gov/news/2475/nasa-climate-modeling-suggests-venus-may-have-been-habitable.
- ↑ McKay, C.; Pollack, J.; Courtin, R. (1991). "The greenhouse and antigreenhouse effects on Titan". Science 253 (5024): 1118–1121. doi:10.1126/science.11538492. PMID 11538492. Bibcode: 1991Sci...253.1118M.
- ↑ Goldblatt, Colin; Watson, Andrew J. (2012). "The Runaway Greenhouse: Implications for Future Climate Change, Geoengineering and Planetary Atmospheres". Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 370 (1974): 4197–4216. doi:10.1098/rsta.2012.0004. PMID 22869797. Bibcode: 2012RSPTA.370.4197G.
- ↑ McKay, C. P.; Pollack, J. B.; Courtin, R. (1991-09-06). "The greenhouse and antigreenhouse effects on Titan" (in en). Science 253 (5024): 1118–1121. doi:10.1126/science.11538492. ISSN 0036-8075. PMID 11538492. Bibcode: 1991Sci...253.1118M.
- ↑ "Titan: Greenhouse and Anti-greenhouse" (in en-US). 2005-11-03. https://www.astrobio.net/retrospections/titan-greenhouse-and-anti-greenhouse/.
Further reading
- Businger, Joost Alois; Fleagle, Robert Guthrie (1980). An introduction to atmospheric physics. International Geophysics (2nd ed.). Academic. ISBN 978-0-12-260355-6. https://books.google.com/books?id=qgWHwX0IpdAC.
- Henderson-Sellers, Ann; McGuffie, Kendal (2005). A climate modelling primer (3rd ed.). Wiley. ISBN 978-0-470-85750-2. https://books.google.com/books?id=9Hc9Ga8UruEC.
External links
 |