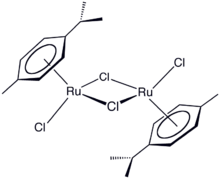
Chemistry:(Cymene)ruthenium dichloride dimer
| |

| |
| Names | |
|---|---|
| Other names
Dichloro(p-cymene)ruthenium(II) dimer
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H28Cl4Ru2 | |
| Molar mass | 612.38 g·mol−1 |
| Appearance | Red solid |
| Melting point | 247 to 250 °C (477 to 482 °F; 520 to 523 K) (decomposes) |
| Slightly, with hydrolysis | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
(Cymene)ruthenium dichloride dimer is the organometallic compound with the formula [(cymene)RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis. The complex is structurally similar to (benzene)ruthenium dichloride dimer.
Preparation and reactions
The dimer is prepared by the reaction of the phellandrene with hydrated ruthenium trichloride.[1] At high temperatures, [(cymene)RuCl2]2 exchanges with other arenes:
- [(cymene)RuCl2]2 + 2 C6Me6 → [(C6Me6)RuCl2]2 + 2 cymene
(Cymene)ruthenium dichloride dimer reacts with Lewis bases to give monometallic adducts:
- [(cymene)RuCl2]2 + 2 PPh3 → 2 (cymene)RuCl2(PPh3)
Such monomers adopt pseudo-octahedral piano-stool structures.
Precursor to catalysts
Treatment of [(cymene)RuCl2]2 with the chelating ligand TsDPENH gives (cymene)Ru(TsDPEN-H), a catalyst for asymmetric transfer hydrogenation.[2]
[(cymene)RuCl2]2 is also used to prepare catalysts (by monomerization with dppf) used in borrowing hydrogen catalysis,[3] a catalytic reaction that is based on the activation of alcohols towards nucleophilic attack.
It can also used to prepare other ruthenium—arene complexes.[4]
References
- ↑ Bennett, M. A.; Huang, T. N.; Matheson, T. W.; Smith, A. K. (1982). "16. (η6 -Hexamethylbenzene)Ruthenium Complexes". (η6-Hexamethylbenzene)ruthenium Complexes. Inorganic Syntheses. 21. pp. 74–78. doi:10.1002/9780470132524.ch16. ISBN 9780470132524.
- ↑ Takao Ikariya; Shohei Hashiguchi; Kunihiko Murata; Ryōji Noyori (2005). "Preparation of Optically Active (R,R)-Hydrobenzoin from Benzoin or Benzil". Organic Syntheses: 10. http://www.orgsyn.org/demo.aspx?prep=v82p0010.
- ↑ Hamid et al.; Advanced Synthesis & Catalysis Volume 349, Issue 10, pages 1555–1575, July 2, 2007; doi:10.1002/adsc.200600638
- ↑ Lautens, M., ed (2001) (in en). Category 1, Organometallics: Compounds with Transition Metal—Carbon π-Bonds and Compounds of Groups 10–8 (Ni, Pd, Pt, Co, Rh, Ir, Fe, Ru, Os). Stuttgart: Georg Thieme Verlag. doi:10.1055/sos-sd-001-00899. ISBN 978-3-13-112131-8. http://www.thieme-connect.de/DOI/DOI?10.1055/b-003-117830.
 |

