Physics:Semipermeable membrane
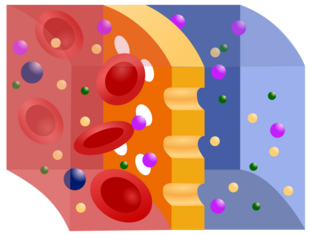
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.[1]
Biological membranes are selectively permeable,[2] with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane.
Biological membranes
An membrane is the lipid bilayer,[2] on which is based the plasma membrane that surrounds all biological cells. A group of phospholipids (consisting of a phosphate head and two fatty acid tails) arranged into a double layer, the phospholipid bilayer is a semipermeable membrane that is very specific in its permeability. The hydrophilic phosphate heads are in the outside layer and exposed to the water content outside and within the cell. The hydrophobic tails are the layer hidden in the inside of the membrane. Cholesterol molecules are also found throughout the plasma membrane and act as a buffer of membrane fluidity.[3] The phospholipid bilayer is most permeable to small, uncharged solutes. Protein channels are embedded in or through phospholipids,[4] and, collectively, this model is known as the fluid mosaic model. Aquaporins are protein channel pores permeable to water.
Cellular communication
Information can also pass through the plasma membrane when signaling molecules bind to receptors in the cell membrane. The signaling molecules bind to the receptors, which alters the structure of these proteins.[5] A change in the protein structure initiates a signalling cascade.[5] The G protein-coupled receptor signalling provides is an important subset of such signalling processes.[6]
Osmotic stress
Due to being semipermeable, the lipid bilayer is subject to osmotic pressure.[7] When the solutes around a cell becomes more or less concentrated, osmotic pressure causes water to flow out of or into the cell to equilibrate.[8] This osmotic stress inhibits cellular functions and molecules, such as DNA, cell membrane assembly, and protein systems, which are dependent on the activity of water in the cell.[9] This can lead to osmotic shock and cell death. Osmoregulation is the method by which cells counteract osmotic stress, and includes osmosensory transporters in the membrane that allow K+ and other molecules to flow through the membrane.[8]
Artificial membranes
Artificial semipermeable membranes see wide usage in research and the medical field. Artificial lipid membranes can easily be manipulated and experimented upon to study biological phenomenon.[10] Other artificial membranes include those involved in drug delivery, dialysis, and bioseparations.[11]
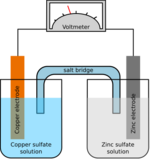
Reverse osmosis
The bulk flow of water through a selectively permeable membrane because of an osmotic pressure difference is called osmosis. This allows only certain particles to go through including water and leaving behind the solutes including salt and other contaminants. In the process of reverse osmosis, water is purified by applying high pressure to a solution and thereby push water through a thin-film composite membrane (TFC or TFM). These are semipermeable membranes manufactured principally for use in water purification or desalination systems. They also have use in chemical applications such as batteries and fuel cells. In essence, a TFC material is a molecular sieve constructed in the form of a film from two or more layered materials. Sidney Loeb and Srinivasa Sourirajan invented the first practical synthetic semi-permeable membrane.[12] Membranes used in reverse osmosis are, in general, made out of polyamide, chosen primarily for its permeability to water and relative impermeability to various dissolved impurities including salt ions and other small molecules that cannot be filtered.
Regeneration of Reverse osmosis membranes
Reverse osmosis membrane modules have a limited life cycle, several studies have endeavored to improve the performance of the process and extend the RO membranes lifespan. However, even with the appropriate pretreatment of the feed water, the membranes lifespan is generally limited to five to seven years.
Discarded RO membrane modules are currently classified worldwide as inert solid waste and are often disposed of in landfills, with limited reuse. Estimates indicated that the mass of membranes annually discarded worldwide reached 12,000 tons. At the current rate, the disposal of RO modules represents significant and growing adverse impacts on the environment, giving rise to the need to limit the direct discarding of these modules.
Discarded RO membranes from desalination operations could be recycled for other processes that do not require the intensive filtration criteria of desalination, they could be used in applications requiring nanofiltration (NF) membranes. [13]
Regeneration process steps:
1- Chemical Treatment
Chemical procedures aimed at removing fouling from the spent membrane; several chemicals agents are used; such as:
- Sodium Hydroxide (alkaline)
- Hydrochloric Acid (Acidic)
- Chelating agents Such as Citric and Oxalic acids
There are three forms of membranes exposure to chemical agents; simple immersion, recirculating the cleaning agent, or immersion in an ultrasound bath.
2 - Oxidative treatment
It includes exposing the membrane to oxidant solutions in order to remove its dense aromatic polyamide active layer and subsequent conversion to a porous membrane. Oxidizing agents such as Sodium Hypochlorite NaClO (10–12%) and Potassium Permanganate KMnO₄ are used.[14] These agents remove organic and biological fouling from RO membranes, They also disinfect the membrane surface, preventing the growth of bacteria and other microorganisms.
Sodium Hypochlorite is the most efficient oxidizing agent in light of permeability and salt rejection solution.
Dialysis tubing
Dialysis tubing is used in hemodialysis to purify blood in the case of kidney failure. The tubing uses a semipermeable membrane to remove waste before returning the purified blood to the patient.[15] Differences in the semipermeable membrane, such as size of pores, change the rate and identity of removed molecules. Traditionally, cellulose membranes were used, but they could cause inflammatory responses in patients. Synthetic membranes have been developed that are more biocompatible and lead to fewer inflammatory responses.[16] However, despite the increased biocompatibility, synthetic membranes have not been linked to decreased mortality.[15]
Other types
Other types of semipermeable membranes are cation-exchange membranes (CEMs), anion-exchange membranes (AEMs), alkali anion exchange membranes (AAEMs) and proton-exchange membranes (PEMs).
References
- ↑ "Osmosis Eggs | Center for Nanoscale Science". Center for Nanoscale Science, Penn State University. https://www.mrsec.psu.edu/content/osmosis-eggs.
- ↑ 2.0 2.1 Caplan, M.J. (2017). "Functional organization of the cell". in Boron, W.F.; Boulpaep, E.L.. Medical physiology (Third ed.). Philadelphia, PA: Elsevier. pp. 8–46. ISBN 9781455743773.
- ↑ Boughter, Christopher T.; Monje-Galvan, Viviana; Im, Wonpil; Klauda, Jeffery B. (2016-11-17). "Influence of Cholesterol on Phospholipid Bilayer Structure and Dynamics" (in en). The Journal of Physical Chemistry B 120 (45): 11761–11772. doi:10.1021/acs.jpcb.6b08574. ISSN 1520-6106. PMID 27771953. https://pubs.acs.org/doi/10.1021/acs.jpcb.6b08574.
- ↑ Friedl, Sarah. "Semipermeable Membranes' Role in Cell Communication - Video & Lesson Transcript" (in en). http://study.com/academy/lesson/semipermeable-membranes-role-in-cell-communication.html.
- ↑ 5.0 5.1 Wood, David. "Semipermeable Membrane: Definition & Overview - Video & Lesson Transcript" (in en). http://study.com/academy/lesson/semipermeable-membrane-definition-lesson-quiz.html.
- ↑ Weis, William I.; Kobilka, Brian K. (20 June 2018). "The Molecular Basis of G Protein–Coupled Receptor Activation". Annual Review of Biochemistry 87 (1): 897–919. doi:10.1146/annurev-biochem-060614-033910. PMID 29925258.
- ↑ Voet, Donald (2001). Fundamentals of Biochemistry (Rev. ed.). New York: Wiley. pp. 30. ISBN 978-0-471-41759-0.
- ↑ 8.0 8.1 Wood, Janet M. (October 2011). "Bacterial Osmoregulation: A Paradigm for the Study of Cellular Homeostasis" (in en). Annual Review of Microbiology 65 (1): 215–238. doi:10.1146/annurev-micro-090110-102815. ISSN 0066-4227. PMID 21663439. https://www.annualreviews.org/doi/10.1146/annurev-micro-090110-102815.
- ↑ Rand*, R. P.; Parsegian, V. A.; Rau, D. C. (2000-07-01). "Intracellular osmotic action" (in en). Cellular and Molecular Life Sciences 57 (7): 1018–1032. doi:10.1007/PL00000742. ISSN 1420-9071. PMID 10961342. https://doi.org/10.1007/PL00000742.
- ↑ Siontorou, Christina G.; Nikoleli, Georgia-Paraskevi; Nikolelis, Dimitrios P.; Karapetis, Stefanos K. (September 2017). "Artificial Lipid Membranes: Past, Present, and Future" (in en). Membranes 7 (3): 38. doi:10.3390/membranes7030038. ISSN 2077-0375. PMID 28933723.
- ↑ Stamatialis, Dimitrios F.; Papenburg, Bernke J.; Gironés, Miriam; Saiful, Saiful; Bettahalli, Srivatsa N. M.; Schmitmeier, Stephanie; Wessling, Matthias (2008-02-01). "Medical applications of membranes: Drug delivery, artificial organs and tissue engineering". Journal of Membrane Science 308 (1): 1–34. doi:10.1016/j.memsci.2007.09.059. ISSN 0376-7388. https://www.sciencedirect.com/science/article/pii/S0376738807007090.
- ↑ Sidney, Loeb & Sourirajan Srinivasa, "High flow porous membranes for separating water from saline solutions", US patent 3133132, published 12 May 1964
- ↑ Lawler, Will; Bradford-Hartke, Zenah; Cran, Marlene J.; Duke, Mikel; Leslie, Greg; Ladewig, Bradley P.; Le-Clech, Pierre (2012-08-01). "Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes". Desalination 299: 103–112. doi:10.1016/j.desal.2012.05.030. ISSN 0011-9164. https://www.sciencedirect.com/science/article/pii/S0011916412002901.
- ↑ Coutinho de Paula, Eduardo; Gomes, Júlia Célia Lima; Amaral, Míriam Cristina Santos (July 2017). "Recycling of end-of-life reverse osmosis membranes by oxidative treatment: a technical evaluation". Water Science and Technology: A Journal of the International Association on Water Pollution Research 76 (3-4): 605–622. doi:10.2166/wst.2017.238. ISSN 0273-1223. PMID 28759443. https://pubmed.ncbi.nlm.nih.gov/28759443/.
- ↑ 15.0 15.1 MacLeod, Alison M; Campbell, Marion K; Cody, June D; Daly, Conal; Grant, Adrian; Khan, Izhar; Rabindranath, Kannaiyan S; Vale, Luke et al. (2005-07-20). Cochrane Kidney and Transplant Group. ed. "Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease" (in en). Cochrane Database of Systematic Reviews 2009 (3): CD003234. doi:10.1002/14651858.CD003234.pub2. PMID 16034894.
- ↑ Kerr, Peter G; Huang, Louis (June 2010). "Review: Membranes for haemodialysis" (in en). Nephrology 15 (4): 381–385. doi:10.1111/j.1440-1797.2010.01331.x. ISSN 1320-5358. PMID 20609086. https://onlinelibrary.wiley.com/doi/10.1111/j.1440-1797.2010.01331.x.
Further reading
- Koros, W. J.; Ma, Y. H.; Shimidzu, T. (1 January 1996). "Terminology for membranes and membrane processes (IUPAC Recommendations 1996)". Pure and Applied Chemistry 68 (7): 1479–1489. doi:10.1351/pac199668071479. See this document for definitions of penetrant (permeant), synthetic (artificial) membrane, and anion-exchange membrane.
- Rozendal, R. A.; Sleutels, T. H. J. A.; Hamelers, H. V. M.; Buisman, C. J. N. (June 2008). "Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater". Water Science and Technology 57 (11): 1757–1762. doi:10.2166/wst.2008.043. PMID 18547927.[non-primary source needed]
- "High Flow Porous Membranes for Separating Water from Saline Solutions US 3133132 A". 12 May 1964. https://www.google.com/patents/US3133132.[non-primary source needed]
External links
- The European Membrane House[yes|permanent dead link|dead link}}], a non-profit international association created to continue the work of the network and parternships developed in NanoMemPro, an earlier EU-funded European network of membrane researchers.
- Short, non-scholarly WiseGeek article, "What is a Semipermeable Membrane.
 |